- 著者
- Shinya Kimura Sota Mori Masashi Yokoya Masamichi Yamanaka
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.70, no.6, pp.443-447, 2022-06-01 (Released:2022-06-01)
- 参考文献数
- 41
- 被引用文献数
- 1
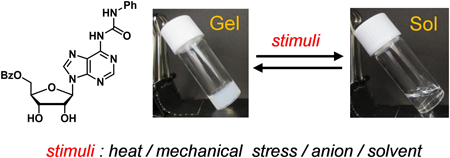
Urea derivatives 1 and 2, synthesized from adenosine, were designed as low-molecular-weight gelators. Hydrophobic groups have been introduced into all or part of the hydroxy groups of the hydrophilic ribose moiety of 1 and 2 to control the solvophilicity of the molecules and their aggregates. Compound 2 selectively formed supramolecular gels in halogenated solvents such as chloroform and 1,2-dichloroethane. The supramolecular gel of 2 and chloroform was thermally stable and its gel-to-sol phase transition temperature was higher than the boiling point of chloroform. The physical properties of the supramolecular gel were investigated by determining its viscoelastic properties using a rheometer. The supramolecular gel realized multiple stimuli-responsive reversible gel–sol phase transitions. The supramolecular gel showed reversible phase transition by repeated warming–cooling cycles accompanying with the gel–sol transitions. The supramolecular gel could undergo five repeated mechano-responsive gel–sol transitions. Gel-to-sol phase transition could also be achieved by adding various anions to the supramolecular gel, such as tetrabutylammonium fluoride. Regelation was realized by adding boron trifluoride etherate to the fluoride ion containing sol. Addition of methanol to the supramolecular gel also induced gel-to-sol phase transition. Regelation was realized by adding molecular sieves 4 Å to the suspension.