- 著者
- Merve Ergül Ahmet Şevki Taşkıran
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.69, no.9, pp.832-839, 2021-09-01 (Released:2021-09-01)
- 参考文献数
- 36
- 被引用文献数
- 9
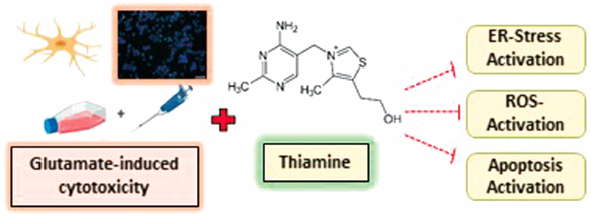
Thiamine (vitamin B1), which is synthesized only in bacteria, fungi and plants and which humans should take with diet, participates in basic biochemical and physiological processes in a versatile way and its deficiency is associated with neurological problems accompanied by cognitive dysfunctions. The rat glioblastoma (C6) model was used, which was exposed to a limited environment and toxicity with glutamate. The cells were stressed by exposure to glutamate in the presence and absence of thiamine. The difference in cell proliferation was evaluated in the XTT assay. Oxidative stress (OS) markers malondialdehyde (MDA), superoxide dismutase (SOD), and catalase (CAT) levels, as well as endoplasmic reticulum (ER) stress markers 78-kDa glucose-regulated protein (GRP78), activating transcription factor-4 (ATF-4), and C/EBP homologous protein (CHOP) levels, were measured with commercial kits. Apoptosis determined by flow cytometry was confirmed by 4′,6-diamidino-2-phenylindole (DAPI) staining. At all concentrations, thiamine protects the cells and increased the viability against glutamate-induced toxicity. Thiamine also significantly decreased the levels of MDA, while increasing SOD and CAT levels. Moreover, thiamine reduced ER stress proteins’ levels. Moreover, it lessened the apoptotic cell amount and enhanced the live-cell percentage in the flow cytometry and DAPI staining. As a result, thiamine may be beneficial nutritional support for individuals with a predisposition to neurodegenerative disorders due to its protective effect on glutamate cytotoxicity in glioblastoma cells by suppressing OS and ER stress.