- 著者
- Rie Ito Chisa Takemura Hiroshi Akiyama Koichi Saito
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.71, no.4, pp.312-317, 2023-04-01 (Released:2023-04-01)
- 参考文献数
- 15
- 被引用文献数
- 1
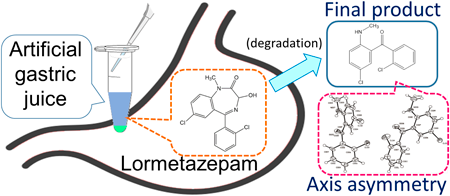
The degradation behavior of three benzodiazepines (BZPs)—lormetazepam (LMZ), lorazepam, and oxazepam—with hydroxy groups on the diazepine ring in artificial gastric juice and the effect of storage pH conditions on drug degradability were monitored using an LC/photodiode array detector (PDA) to estimate their pharmacokinetics in the stomach. Although the three BZPs degraded in artificial gastric juice, none could be restored, despite increasing the storage pH, implying that the degradation reaction was irreversible. As for LMZ, we discussed the physicochemical parameters, such as the activation energy and activation entropy involved in the degradation reaction as well as the reaction kinetics; one of the degradation products was isolated and purified for structural analysis. In the LMZ degradation experiment, peaks corresponding to degradation products, (A) and (B), were detected through the LC/PDA measurements. Regarding the degradation behavior, we hypothesized that LMZ was degraded into (B) via (A), where (A) was an intermediate and (B) was the final product. Although the isolation of degradation product (A) was challenging, degradation product (B) could be isolated and was confirmed to be “methanone, [5-chloro-2-(methylamino)phenyl](2-chlorophenyl)-” based on structure determination using various instrumental analyses. The compound exhibited axis asymmetry as determined using single-crystal X-ray structure analysis. Because the formation of degradation product (B) was irreversible, it would be prudent to target the final degradation product (B) and LMZ for identification when detecting LMZ in human stomach contents, such as during forensic dissection.
- 著者
- Naoki TANAKA Riki GOTO Rie ITO Miho HAYAKAWA Taketoshi OGAWA Koichi FUJIMOTO
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.46, no.4, pp.639-646, 1998-04-15 (Released:2008-03-31)
- 参考文献数
- 14
- 被引用文献数
- 2 2
A series of [2-(ω-phenylalkyl)phenoxy]alkylamines was synthesized and their 5-hydroxytryptamine2 (5-HT2) and/or dopamine2 (D2) receptor antagonistic activities were examined in vitro. [2-(4-Phenylbutyl)phenoxy]alkylamines showed strong inhibition of both 5-HT2 and D2 receptors. It particular, [2-(4-phenylbutyl)phenoxy]-methylpiperidine derivatives, 10b, 10i and 10q, exhbited potent inhibition. The structure-activity relationships in this series of compounds are discussed.