- 著者
- Ryo Yazaki
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.69, no.6, pp.516-525, 2021-06-01 (Released:2021-06-01)
- 参考文献数
- 88
- 被引用文献数
- 5
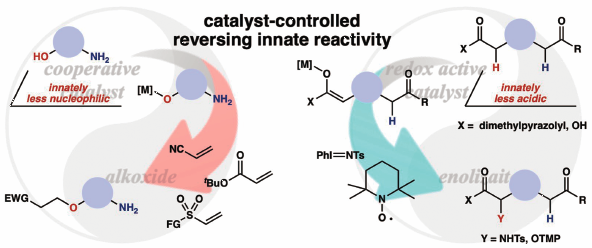
Catalytic chemoselective reactions of innately less reactive functionalities over more reactive functionalities are described. A cooperative catalyst comprising a soft Lewis acid/hard Brønsted base enabled chemoselective activation of a hydroxyl group over an amino group, allowing for nucleophilic addition to electron-deficient olefins. The reaction could be applicable for a variety of amino alcohols, including pharmaceuticals, without requiring a tedious protection–deprotection process. Chemoselective enolization and subsequent α-functionalization of carboxylic acid derivatives were also achieved by a redox active catalyst through the radical process, providing unnatural α-amino/hydroxy acid derivatives bearing a complex carbon framework and a diverse set of functionalities. The present chemoselective catalysis described herein offers new opportunities to expand the chemical space for innovative drug discovery research.