- 著者
- Ryoichi TATARA Yukihiro OKAMOTO Yosuke UGATA Kazuhide UENO Masayoshi WATANABE Kaoru DOKKO
- 出版者
- The Electrochemical Society of Japan
- 雑誌
- Electrochemistry (ISSN:13443542)
- 巻号頁・発行日
- vol.89, no.6, pp.590-596, 2021-11-05 (Released:2021-11-05)
- 参考文献数
- 43
- 被引用文献数
- 3
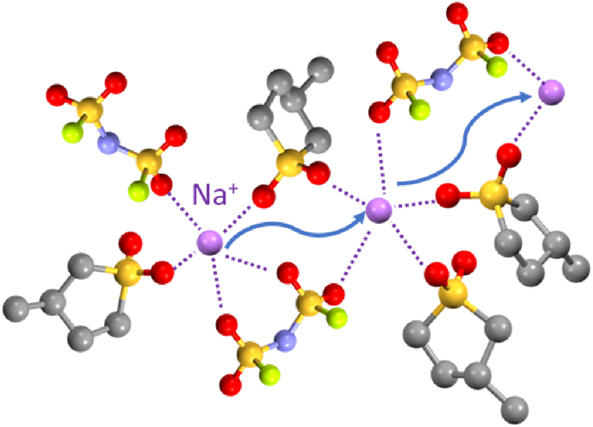
The performance of a sodium-ion (Na) battery is significantly influenced by its electrolyte characteristics. In particular, the transport properties of the electrolyte have considerable effects on the discharge rate capability. During discharging of a Na battery at high current densities, a concentration gradient of Na salt develops because both cations and anions are mobile in the liquid electrolyte. Concentration polarization can be suppressed by increasing the Na+ transference number (tNa+) of the electrolyte. This study demonstrates that highly concentrated NaN(SO2F)2 dissolved in 3-methylsulfolane (MSL) exhibits a high tNa+ value of >0.6 under anion-blocking conditions. Raman spectroscopy revealed that Na+ ions formed complexes with MSL and anions in the electrolyte. Na+ ions exchange ligands dynamically and move faster than the ligands, resulting in a high tNa+. The high tNa+ enables a high-rate discharge of the Na battery, despite the low ionic conductivity of the highly concentrated electrolyte.