- 著者
- Yutaka Matsuda Brian A. Mendelsohn
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.69, no.10, pp.976-983, 2021-10-01 (Released:2021-10-01)
- 参考文献数
- 78
- 被引用文献数
- 35
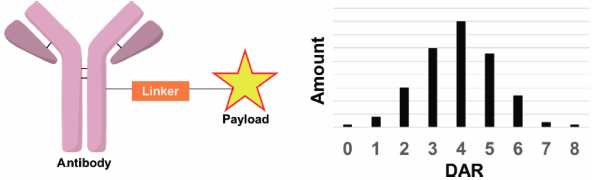
Antibody–drug conjugates (ADCs) are biopharmaceuticals produced by chemically linking small molecules (payloads) to antibodies that possess specific affinity for the target cell. The ADCs currently on the commercially market are the result of a stochastic conjugation of highly-potent payloads to multiple sites on the monoclonal antibody, resulting in a heterogeneous drug–antibody ratio (DAR) and drug distribution. The heterogeneity inherent to ADCs not produced site-specifically may not only be detrimental to the quality of the drug but also is less-desirable from the perspective of regulatory science. An ideal method or unified approach used to measure the DAR for ADCs, a critical aspect of their analysis and characterization, has not yet been established in the ADC field and remains an often-challenging issue for bioanalytical chemists. In this review we describe, compare, and evaluate the characteristics of various DAR determination methods for ADCs featuring recently reported technologies. The future landscape of bioconjugate DAR analysis is also discussed.