- 著者
- Kouya Yamaki Kiyoe Ohta Norihiro Kobayashi Izumi Morita Yuki Kiguchi Hiroyuki Oyama Ken Ito Asuka Nanbo Hirozo Oh-oka Yutaka Koyama Yoshiki Kawata Hirotaka Fujisawa Mitsuhiro Ohta
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.45, no.8, pp.1022-1026, 2022-08-01 (Released:2022-08-01)
- 参考文献数
- 20
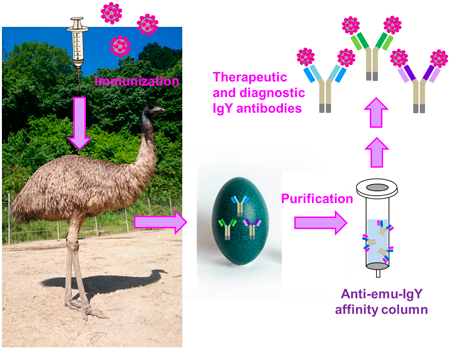
The emu is the second largest ratite; thus, their sera and egg yolks, obtained after immunization, could provide therapeutic and diagnostically important immunoglobulins with improved production efficiency. Reliable purification tools are required to establish a pipeline for supplying practical emu-derived antibodies, the majority of which belongs to the immunoglobulin Y (IgY) class. Therefore, we generated a monoclonal secondary antibody specific to emu IgY. Initially, we immunized an emu with bovine serum albumin multiply haptenized with 2,4-dinitrophenyl (DNP) groups. Polyclonal emu anti-DNP antibodies were partially purified using conventional precipitation method and used as antigen for immunizing a BALB/c mouse. Splenocytes were fused with myeloma cells and a hybridoma clone secreting a desirable secondary antibody (mAb#2-16) was established. The secondary antibody bound specifically to emu-derived IgY, distinguishing IgYs from chicken, duck, ostrich, quail, and turkey, as well as human IgGs. Affinity columns immobilizing the mAb#2-16 antibodies enabled purification of emu IgY fractions from sera and egg yolks via simple protocols, with which we succeeded in producing IgYs specific to the severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) spike protein with a practical binding ability. We expect that the presented purification method, and the secondary antibody produced in this study, will facilitate the utilization of emus as a novel source of therapeutic and diagnostic antibodies.
1 0 0 0 OA Antibody Fragments for On-Site Testing of Cannabinoids Generated via in Vitro Affinity Maturation
- 著者
- Izumi Morita Hiroyuki Oyama Mayumi Yasuo Kazuhisa Matsuda Kengo Katagi Aya Ito Hiroka Tatsuda Hiroyuki Tanaka Satoshi Morimoto Norihiro Kobayashi
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.40, no.2, pp.174-181, 2017-02-01 (Released:2017-02-01)
- 参考文献数
- 40
- 被引用文献数
- 10 17
Law enforcement against illicit use of cannabis and related substances requires rapid, feasible, and reliable tools for on-site testing of cannabinoids. Notably, methods based on cannabinoid-specific antibodies enable efficient screening of multiple specimens. Antibody engineering may accelerate development of modern and robust testing systems. Here, we used in vitro affinity maturation to generate a single-chain Fv fragment (scFv) that recognizes with high affinity the psychoactive cannabinoid, Δ9-tetrahydrocannabinol (THC). A mouse monoclonal antibody against THC, Ab-THC#33, with Ka 6.2×107 M−1 (as Fab fragment) was established by the hybridoma technique. Then, a “wild-type” scFv (wt-scFv) with Ka, 1.1×107 M−1 was prepared by bacterial expression of a fusion gene combining the VH and VL genes for Ab-THC#33. Subsequently, random point mutations in VH and VL were generated separately, and the resulting products were assembled into mutant scFv genes, which were then phage-displayed. Repeated panning identified a mutant scFv (scFv#m1-36) with 10-fold enhanced affinity (Ka 1.1×108 M−1) for THC, in which only a single conservative substitution (Ser50Thr) was present at the N-terminus of the VH-complementarity-determining region 2 (CDR2) sequence. In competitive enzyme-linked immunosorbent assay (ELISA), the mutant scFv generated dose–response curves with midpoint 0.27 ng/assay THC, which was 3-fold lower than that of wt-scFv. Even higher reactivity with a major THC metabolite, 11-nor-9-carboxy-Δ9-tetrahydrocannabinol, indicated that the mutant scFv will be useful for testing not only THC in confiscated materials, but also the metabolite in urine. Indeed, the antibody fragment is potentially suitable for use in advanced on-site testing platforms for cannabinoids.