- 著者
- Keisuke Kinoshita Miyuki Yamaguchi Hirohisa Sasou Hideyuki Konishi Kei Manabe
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.71, no.2, pp.175-182, 2023-02-01 (Released:2023-02-01)
- 参考文献数
- 51
- 被引用文献数
- 1
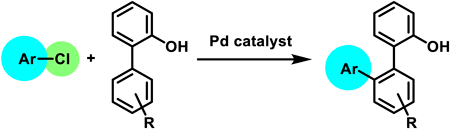
Palladium-catalyzed, hydroxy-group-directed C–H arylation of [1,1′-biphenyl]-2-ols with chloroarenes was performed. The reaction showed a broad substrate scope and was successfully applied to pharmaceuticals containing a chloro group. Using 2-heteroarylphenols instead of [1,1′-biphenyl]-2-ols also yielded the desired products. The arylated product was further transformed into a triphenylene derivative.
- 著者
- Hideyuki Konishi Tomoyuki Sekino Kei Manabe
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- pp.c18-00042, (Released:2018-02-14)
- 参考文献数
- 33
- 被引用文献数
- 7
A practical Pd-catalyzed carbonylation of (hetero)aryl bromides using a crystalline carbon monoxide (CO) surrogate, 2,4,6-trichlorophenyl formate (TCPF), was developed. This reaction proceeds without the slow addition technique that was previously required and with a low catalyst loading (1 mol%). The utility of this Pd-catalyzed external-CO-free carbonylation using TCPF was demonstrated in the synthesis of a histone deacetylase inhibitor.
1 0 0 0 OA Practical Synthesis of Axially Chiral Dicarboxylates via Pd-Catalyzed External-CO-Free Carbonylation
- 著者
- Hideyuki Konishi Fumika Hoshino Kei Manabe
- 出版者
- 公益社団法人日本薬学会
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.64, no.10, pp.1438-1441, 2016-10-01 (Released:2016-10-01)
- 参考文献数
- 35
- 被引用文献数
- 13
We have developed a safe and practical synthetic method for preparing axially chiral diphenyl dicarboxylates using Pd-catalyzed external-CO-free carbonylation with phenyl formate as a CO surrogate. Optimized conditions consisted of axially chiral [1,1′-binaphthalene]-2,2′-diyl ditriflate and its congeners, each easily prepared from commercially available enantiomerically pure diols, Pd(OAc)2, 1,3-bis(diphenylphosphino)propane, ethyldiisopropylamine, and no solvent. To demonstrate the potential utility of these products, this method was conducted on gram-scale and the phenyl ester products were converted to other useful compounds, and both processes were carried out without difficulty.