- 著者
- Airi YAMAGUCHI Naoki SHIDA Mahito ATOBE Tomoko YAJIMA
- 出版者
- The Electrochemical Society of Japan
- 雑誌
- Electrochemistry (ISSN:13443542)
- 巻号頁・発行日
- vol.91, no.11, pp.112016, 2023-11-30 (Released:2023-11-30)
- 参考文献数
- 35
- 被引用文献数
- 1
Photocatalytic single-electron transfer (SET) reactions involving perfluoroalkyl halides play a crucial role in synthetic organic chemistry. However, the electrochemical data for these compounds, which are essential in the discussion of the SET process, are missing. In this study, the electrochemical reduction potentials of perfluoroalkyl halides, alkyl halides, and other analogous compounds were investigated in 0.1 M Bu4NPF6/CH3CN using Ag, Pt, and glassy carbon electrodes. The Ag electrode showed remarkable catalytic properties and a positive reduction peak shift during the reduction reaction; this indicates that the Ag electrode is suitable for estimating the electrochemical potential of the SET process. This study provides a comprehensive dataset for the electrochemical measurements of perfluoroalkyl and alkyl halides, which will help synthetic organic chemists select appropriate reaction systems for these compounds.
- 著者
- Kazuhiro OKAMOTO Yasushi IMADA Naoki SHIDA Yoshikazu KITANO Mahito ATOBE Kazuhiro CHIBA
- 出版者
- The Electrochemical Society of Japan
- 雑誌
- Electrochemistry (ISSN:13443542)
- 巻号頁・発行日
- vol.91, no.11, pp.112006, 2023-11-28 (Released:2023-11-28)
- 参考文献数
- 21
- 被引用文献数
- 1
Herein, we report that the 2,7-dimethoxynaphthyl (2,7-DMN) group is a novel electroauxiliary that is readily installed at the N-α position of a carbamate through Friedel–Crafts-type arylation. The resulting N-α C–C bond is easily cleaved through low-potential electrochemical oxidation to give the corresponding iminium cation.
- 著者
- Rumi IZUMIYA Mahito ATOBE Naoki SHIDA
- 出版者
- The Electrochemical Society of Japan
- 雑誌
- Electrochemistry (ISSN:13443542)
- 巻号頁・発行日
- vol.91, no.11, pp.112003, 2023-11-28 (Released:2023-11-28)
- 参考文献数
- 19
- 被引用文献数
- 1
β-Scission from alkoxy radical enables selective Csp3-Csp3 bond cleavage under ambient conditions, offering a useful method for organic synthesis. Various photocatalytic systems for β-scission have been reported, where proton-coupled electron transfer (PCET) mechanism plays a key role in the generation of alkoxy radical and thus β-scission. Electrochemical β-scission has been mainly pioneered in the presence of mediator, and a direct electrochemical system has rarely been investigated. Here, we investigated the β-scission via direct electrochemical oxidation using a model compound with β-O-4 linkage. Synthetic experiments suggested smooth progress of β-scission in the presence of collidine as a base. Cyclic voltammetry measurement, voltammetric simulation, and quantum simulation suggested the PCET mechanism is responsible for the electrochemical reaction, which is followed by β-scission process. This report provides fundamental insights into the electrochemical β-scission via direct electron transfer on the electrode, which contribute to future applications such as biomass valorization.
- 著者
- Shohei YOSHINAGA Mahito ATOBE Naoki SHIDA
- 出版者
- The Electrochemical Society of Japan
- 雑誌
- Electrochemistry (ISSN:13443542)
- 巻号頁・発行日
- vol.91, no.11, pp.112002, 2023-11-28 (Released:2023-11-28)
- 参考文献数
- 14
Redox behavior is a fundamental and fascinating feature of polycyclic aromatic hydrocarbons (PAHs). Cyclic voltammetry (CV) measurements are commonly performed to estimate the electronic structure of PAHs and to determine the stability of their oxidation and reduction states. However, the influences of electrolytes on electrochemically oxidized/reduced PAHs have rarely been discussed. In this note, we report voltammetric analyses of five PAHs (anthracene, 9,10-dimethylanthracene, phenanthrene, pyrene, and perylene) in Bu4NB(C6F5)4/CH2Cl2 and Bu4NTfO/CH2Cl2, respectively, to highlight how the electrolyte-coordination affects the oxidative voltammetric behavior of PAHs. In most cases, reversible voltammetric responses were obtained with Bu4NB(C6F5)4/CH2Cl2, suggesting that this electrolyte is enough weakly coordinating to investigate its intrinsic oxidation behavior. On the other hand, irreversible voltammetric responses were obtained with Bu4NTfO/CH2Cl2, indicating that the presence of a relatively coordinating anion, TfO−, destabilizes the radical cation species and induces further chemical and electrochemical processes. This study provides hints for rational electrolyte design to properly understand the redox behavior of molecules and maximize the potential of functional molecules for applications related to redox chemistry.
- 著者
- Yuto NAKAMURA Yasushi SATO Naoki SHIDA Mahito ATOBE
- 出版者
- The Electrochemical Society of Japan
- 雑誌
- Electrochemistry (ISSN:13443542)
- 巻号頁・発行日
- pp.21-00053, (Released:2021-05-12)
- 参考文献数
- 27
- 被引用文献数
- 1
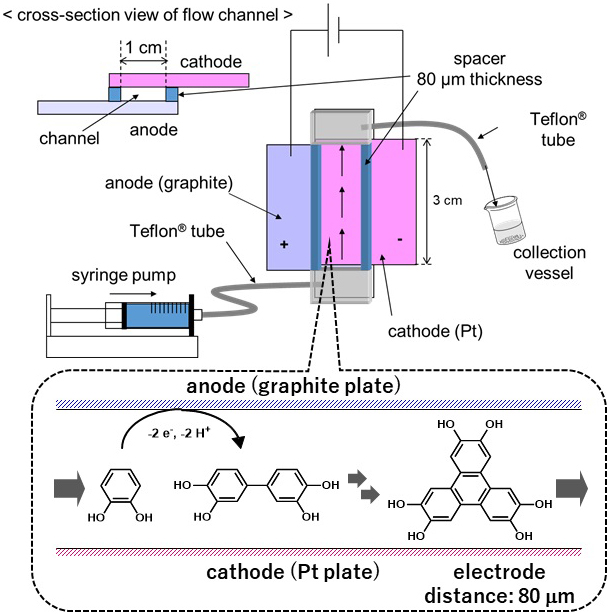
In this study, we report one step electrochemical trimerization of catechol to 2,3,6,7,10,11-hexahydroxytriphenylene (HHTP) for the first time. Electrochemical trimerization was demonstrated in a flow microreactor, which offers advantages for reaction screening owing to short reaction time and small reaction scale, as well as avoiding the further oxidation of HHTP. The use of 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) as a solvent was essential for the efficient production of HHTP. Computational simulation, pKa calculation, and electrochemical measurements gave some important insights into the mechanism of the electrochemical oxidation of catechol in HFIP.