- 著者
- Norihiro Togo Hirotaka Murase Jeongsu Lee Yosuke Taniguchi Shigeki Sasaki
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.70, no.7, pp.498-504, 2022-07-01 (Released:2022-07-01)
- 参考文献数
- 17
- 被引用文献数
- 2
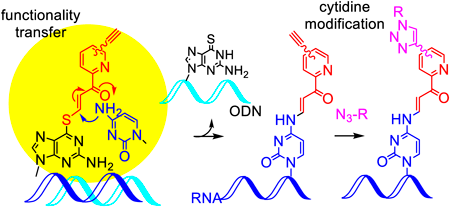
Due to the importance of the RNA chemical modifications, methods for the selective chemical modification at a predetermined site of the internal position of RNA have attracted much attention. We have developed functional artificial nucleic acids that modify a specific site of RNA in a site- and base-selective manner. In addition, the copper-catalyzed azide-alkyne cycloaddition (CuAAC) has been shown to introduce additional molecules on the alkynes attached to the pyridine ring. However, it was found that some azide compounds produced the cycloadduct in lower yields. Therefore, in this study, we synthesized the pyridinyl transfer group with the alkyne attached via a polyethylene glycol (PEG) linker with a different length and optimized its structure for both the transfer and CuAAC reaction. Three new transfer groups were synthesized by introducing an alkyne group at the end of the triethylene (11), tetraethylene (12) or pentaethylen glycol linker (13) at the 5-position of the pyridine ring of (E)-3-iodo-1-(pyridin-2-yl)prop-2-en-1-one. These transfer groups were introduced to the 6-thioguanine base in the oligodeoxynucleotide (ODN) in high yields. The transfer groups 11 and 12 more efficiently underwent the cytosine modification. For the CuAAC reaction, although 7 showed low adduct yields with the anionic azide compound, the new transfer groups, especially 12 and 13, significantly improved the yields. In conclusion, the transfer groups 12 and 13 were determined to be promising compounds for the modification of long RNAs.