- 著者
- Yohei Kawano Maho Katsuyama Masashi Nagata Maki Obana Satoshi Nakamatsu Ayano Mori Namiki Sakamoto Yasunari Mano Kenichi Negishi Shuji Shimada Takao Aoyama
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.44, no.2, pp.238-244, 2021-02-01 (Released:2021-02-01)
- 参考文献数
- 28
- 被引用文献数
- 4
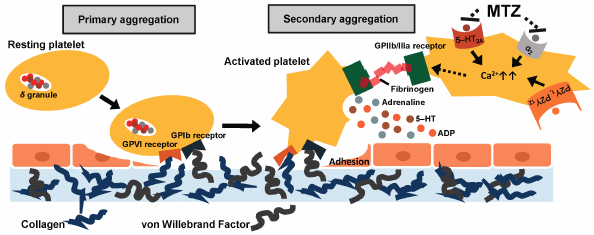
Mirtazapine (MTZ) is a noradrenergic and specific serotonergic antidepressant. MTZ is reportedly associated with an increased risk of bleeding. However, the underlying mechanism remains unclear. In this study, we investigated the antiplatelet effect of MTZ in mice via light transmission aggregometry to elucidate the mechanism of MTZ-induced bleeding. The results of the ex vivo study showed that the oral administration of MTZ (20 or 100 mg/kg) significantly suppressed platelet aggregation mediated by the synergic interaction of 5-hydroxytryptamine (5-HT) and adrenaline. Additionally, MTZ significantly suppressed platelet aggregation, mediated by the synergic interaction of ADP and 5-HT or adrenaline. Similar results were obtained in vitro, under the condition of 5-HT- and adrenaline-induced platelet aggregation. Overall, the results suggest that MTZ exerts antiplatelet effect by co-blocking 5-HT2A and α2-adrenergic receptors on platelets and suppresses platelet aggregation mediated by ADP, increased by either 5-HT or adrenaline. Thus, a detailed monitoring of bleeding is recommended for patients taking MTZ.
3 0 0 0 OA Comprehensive Exploration of Medications That Affect the Bleeding Risk of Oral Anticoagulant Users
- 著者
- Yohei Kawano Masashi Nagata Saeko Nakamura Yuuki Akagi Tatsunori Suzuki Emi Tsukada Mai Hoshiko Azusa Kujirai Satoshi Nakamatsu Tomoki Nishikawa Aya Enomoto Kenichi Negishi Shuji Shimada Takao Aoyama Yasunari Mano
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.44, no.5, pp.611-619, 2021-05-01 (Released:2021-05-01)
- 参考文献数
- 41
- 被引用文献数
- 2
Oral anticoagulants (OACs) pose a major bleeding risk, which may be increased or decreased by concomitant medications. To explore medications that affect the bleeding risk of OACs, we conducted a nested case-control study including 554 bleeding cases (warfarin, n = 327; direct OACs [DOACs], n = 227) and 1337 non-bleeding controls (warfarin, n = 814; DOACs, n = 523), using a Japanese health insurance database from January 2005 to June 2017. Major bleeding risk associated with exposure to concomitant medications within 30 d of the event/index date was evaluated, and adjusted odds ratios (aORs) were calculated using logistic regression analysis. Several antihypertensive drugs, such as amlodipine and bisoprolol, were associated with a decreased risk of bleeding (warfarin + amlodipine [aOR, 0.64; 95% confidence interval (CI): 0.41–0.98], DOACs + bisoprolol [aOR, 0.51; 95% CI, 0.33–0.80]). As hypertension is considered a significant risk factor for intracranial bleeding in antithrombotic therapy, antihypertensive drugs may suppress intracranial bleeding. In contrast, telmisartan, a widely used antihypertensive drug, was associated with an increased risk of bleeding [DOACs + telmisartan (aOR, 4.87; 95% CI, 1.84–12.91)]. Since telmisartan is an inhibitor of P-glycoprotein (P-gp), the elimination of rivaroxaban and apixaban, which are substrates of P-gp, is hindered, resulting in increased blood levels of both drugs, thereby increasing the risk of hemorrhage. In conclusion, antihypertensive drugs may improve the safety of OACs, and the pharmacokinetic-based drug interactions of DOACs must be considered.