- 著者
- Seiya Ohki Shingo Ogawa Hiroki Takano Hayato Shimazaki Momoka Fukae Tomomi Furihata Hiromi Shibasaki Akitomo Yokokawa
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.46, no.5, pp.736-740, 2023-05-01 (Released:2023-05-01)
- 参考文献数
- 22
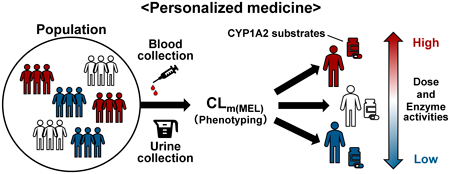
The method of administering caffeine as a probe to evaluate the phenotypic activity of the CYP1A2, has not yet been applied clinically. In contrast, if endogenous melatonin (MEL) metabolism can be used to assess CYP1A2 activity, it could be a simple method that does not require substance administration. The study aim was to calculate the MEL partial metabolic clearance (CLm(MEL)) from plasma MEL and its urinary metabolites and to test the potential of this approach as a novel CYP1A2 phenotyping method. Nine subjects were included in the study; 3 had 6 blood and 4 urine samples collected between 10:00 and 18:00 (collectively, the intraday sample). Nine subjects had 3 blood samples and 2-h urine samples collected between 10:00 and 12:00 once a week for 3 weeks (interday sample). The CLm(MEL) was calculated from the plasma area under the curve (AUC) of MEL (AUCMEL) and urinary MEL metabolites excretion (X6MEL). Among the intraday samples, the AUCMEL ranged from 6.45–13.17 pmol·h/L and X6MEL ranged from 0.204–0.899 nmol/2 h, showing a decrease in concentration over time. In contrast, the CLm(MEL) ranged from 30.52–69.57 L/h (within-individual percent relative standard deviation: 9.2–20.1%), showing no time-dependent variation. Large interindividual variability was observed in AUCMEL and X6MEL in the interday sample, but CLm(MEL) showed small interindividual variabilities. The CLm(MEL) was 1.8-fold higher for smokers than for nonsmokers. The results obtained in this study may be valuable in future studies of evaluating novel CYP1A2 phenotyping method.
- 著者
- Seiya Ohki Miyu Kunimatsu Shingo Ogawa Hiroki Takano Tomomi Furihata Hiromi Shibasaki Akitomo Yokokawa
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.70, no.5, pp.375-382, 2022-05-01 (Released:2022-05-01)
- 参考文献数
- 27
- 被引用文献数
- 2
Evaluation of endogenous melatonin (MEL) secretion using its urinary metabolites is useful for the treatment of circadian rhythm sleep disorders. The primary melatonin metabolites excreted in the urine are 6-hydroxymelatonin (6-O-MEL) sulfate (S-O-MEL) and 6-O-MEL glucuronate, which result from sequential MEL metabolism by phases I and II drug metabolizing enzymes. To determine the accurate MEL secretion level, these urinary metabolites should be enzymatically deconjugated and converted into MEL. Furthermore, the use of LC–tandem mass spectrometry (LC–MS/MS) is preferable for the precision of this determination. Therefore, as part of our ongoing efforts to ultimately determine the level of MEL secretion, we herein aimed to develop an LC–MS/MS-based quantification method for 6-O-MEL and optimize deconjugation conditions. We determined the LC–MS/MS conditions of 6-O-MEL measurement and optimized the conditions of enzymatic reactions. The most efficient S-O-MEL deconjugation (102.1%) was achieved with Roche Glucuronidase/Arylsulfatase (from Helix pomatia) at 37 °C, pH-4.0 reaction buffer, and 60 min of reaction time. For human urine samples, the minimum amount of the enzyme required was 5944 units. Under these conditions, the accuracy and precision values of the 6-O-MEL determination (relative errors and standard deviation) were −3.60–−0.47% and <6.80%, respectively. Finally, we analyzed the total amount of MEL metabolites excreted in 24-h urine samples; it was 6.70–11.28 µg in three subjects, which is comparable with the values reported till date. Thus, we have established a new method of measuring the total 6-O-MEL in human urine samples using an LC–MS/MS coupled with the prerequisite deconjugation reaction.
- 著者
- Keita Kitamura Kenta Umehara Ryo Ito Yoshiyuki Yamaura Takafumi Komori Hanae Morio Hidetaka Akita Tomomi Furihata
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.44, no.7, pp.984-991, 2021-07-01 (Released:2021-07-01)
- 参考文献数
- 35
- 被引用文献数
- 9
In vitro blood–brain barrier (BBB) models are essential research tools for use in developing brain-targeted drugs and understanding the physiological and pathophysiological functions of the BBB. To develop BBB models with better functionalities, three-dimensional (3D) culture methods have gained significant attention as a promising approach. In this study, we report on the development of a human conditionally immortalized cell-based multicellular spheroidal BBB (hiMCS-BBB) model. After being seeded into non-attachment culture wells, HASTR/ci35 (astrocytes) and HBPC/ci37 cells (brain pericytes) self-assemble to form a spheroid core that is then covered with an outer monolayer of HBMEC/ci18 cells (brain microvascular endothelial cells). The results of immunocytochemistry showed the protein expression of several cellular junction and BBB-enriched transporter genes in HBMEC/ci18 cells of the spheroid model. The permeability assays showed that the hiMCS-BBB model exhibited barrier functions against the penetration of dextran (5 and 70 kDa) and rhodamine123 (a P-glycoprotein substrate) into the core. On the other hand, facilitation of 2-(N-[7-nitrobenz-2-oxa-1,3-diazol-4-yl]amino)-2-deoxyglucose (2-NBDG; a fluorescent glucose analog) uptake was observed in the hiMCS-BBB model. Furthermore, tumor necrosis factor-alpha treatment elicited an inflammatory response in HBMEC/ci18 cells, thereby suggesting that BBB inflammation can be recapitulated in the hiMCS-BBB model. To summarize, we have developed an hiMCS-BBB model that possesses fundamental BBB properties, which can be expected to provide a useful and highly accessible experimental platform for accelerating various BBB studies.