1 0 0 0 OA 銀メッキ銅線の赤疹腐食
- 著者
- P. L. Anthony O. M. Brown 福谷 英二
- 出版者
- Japan Society of Corrosion Engineering
- 雑誌
- 防蝕技術 (ISSN:00109355)
- 巻号頁・発行日
- vol.14, no.9, pp.412-417, 1965-09-15 (Released:2009-11-25)
- 参考文献数
- 4
1 0 0 0 OA 市販純チタンと比較したチタン合金の耐食性
- 著者
- D. Schlain C. B. Kenahan 大塚 陸郎
- 出版者
- Japan Society of Corrosion Engineering
- 雑誌
- 防蝕技術 (ISSN:00109355)
- 巻号頁・発行日
- vol.8, no.6, pp.260-263, 1959-06-25 (Released:2009-11-25)
- 参考文献数
- 7
1 0 0 0 OA 酸洗浄時における銅合金の腐食について
- 著者
- 福井 三郎 川野 進 永江 康雄
- 出版者
- Japan Society of Corrosion Engineering
- 雑誌
- 防蝕技術 (ISSN:00109355)
- 巻号頁・発行日
- vol.11, no.5, pp.198-201, 1962-05-15 (Released:2009-11-25)
- 参考文献数
- 9
During the acid cleaning of various equipments, one of the troubles frequently encountered is acid attack on construction materials. Therefore, care should be taken to protect the equipment from acid attack during acid cleaning.Although the acid attack on steel has been studied for many years, there seems to be very little study concerning the acid attack on copper alloys, because the corrosion rates of copper alloys are considered to be comparatively low in the non-oxidizing acid solutions such as hydrochloric acid.However, the acid solution containing oxidizing agents such as oxygen, ferric or cupric ion, etc. may cause attack.In this study, we have investigated the effects of acid concentration, temperature, gas atmosphere and oxidizing ions such as ferric or cupric ion on the corrosion of copper alloys in hydrochloric acid solutions.As a result, it has been found that in the presence of oxidizing ions, the inhibition effect of inhibitors is markedly decreased, and the corrosion rates of copper alloys are greatly accelerated in the inhibited hydrochloric acid solution.The tendency is more remarkable when air is bubbled through the solution.On the hand, in the absence of oxidizing ions, the corrosion of copper alloys in hydrochloric acid can be prevented by inhibitors even when air is bubbled through the solution.
1 0 0 0 OA アルミニウムおよび陽極化成アルミニウムと軟鋼およびステンレス鋼との組合せの接触腐食
- 著者
- 田島 栄 森 健実 小宮 衛
- 出版者
- Japan Society of Corrosion Engineering
- 雑誌
- 防蝕技術 (ISSN:00109355)
- 巻号頁・発行日
- vol.14, no.3, pp.109-117, 1965-03-15 (Released:2009-11-25)
- 参考文献数
- 12
Contact corrosion of couples of aluminum-mild steel, aluminum-stainless steel, anodized aluminummild steel, and anodized aluminum-stainless steel was studied. Corrosion current, weight loss, polarization measurement and salt spray test were carried out on these couples in various inorganic corrosive media: 1N NaCl, 0.1N NaCl, 1N CaCl2, 0.1N CaCl2, 1N Na2SO4, satd. Ca(OH)2 (pH=12.5), 0.38N Na2CO3, 0.5N Na2CO3, 0.1N NaCl, 0.069N NH4OH, 0.09N H2SO4, 0.1N HCl.Under various experimental conditions, both aluminum and anodized aluminum were in general less noble than mild steel and stainless steel except in sodium sulfate and sulfuric acid solutions, in which mild steel was anodic against aluminum and anodized aluminum.In most of the experimental conditions, aluminum and anodized aluminum sacrificially protected mild steel and stainless steel from corrosion completely. Weight loss of aluminum was, however, much greater than that of anodized aluminum. Corrosion current by the aluminum-mild steel and aluminum-stainless steel couples in alkaline solutions was about ten to one hundred times larger than in neutral solutions, while, in the case of anodized aluminum-mild steel and anodized aluminum-stainless steel couples, except in neutral and acidic solutions containing Cl ion, only a small corrosion current was measured.
1 0 0 0 ユーフィンヒーター銅管の異常腐蝕について
- 著者
- 岡野 一良 北村 義治 高橋 礼治
- 出版者
- Japan Society of Corrosion Engineering
- 雑誌
- 防蝕技術 (ISSN:00109355)
- 巻号頁・発行日
- vol.4, no.1, pp.27-30, 1955
An U-fin heater used as an air heater for drying powder materials was damaged due to corrosion of the heating copper tubes. As the results of experiments, it was concluded that the corrosion was caused by the adhesion of solder to inner surface of the copper tube. In order to remove the solder, some methods both by chemicals and by electrolysis were examined. Although we have not yet found a method, which removes the solder completely and not affects copper tubes, we obtained some promising method, especially one by chemicals.
1 0 0 0 OA ステンレス鋼の応力腐食割れに及ぼす温度の影響
- 著者
- 木島 茂
- 出版者
- Japan Society of Corrosion Engineering
- 雑誌
- 防蝕技術 (ISSN:00109355)
- 巻号頁・発行日
- vol.17, no.9, pp.381-388, 1968-09-15 (Released:2009-11-25)
- 参考文献数
- 24
- 被引用文献数
- 7 1
Arrhenius plots were made using data on uniaxially stressed specimens of Types 304, 303 Se, 201, and 431 stainless steels immersed in 35% MgCl2 solution at various temperatures, pH of which had been adjusted to 3 at 22°C by adding HCl. The reciprocal of time-to-failure was taken as the rate of stress-corrosion cracking. Although this is not exactly correct, the results were in good agreement with the Arrhenius' equation. Furthermore, it was found that both the activation energy and logarithm of the rate coefficient, i. e. the pre-exponential term, increased in direct proportion to the stress-level applied and, in addition, the lines illustrating the above relations breaked at a stress-level corresponding to 0.03% proof yield strength of Types 304 and 303 Se steels. As for the other steels such a break was not evidently observed.Arrhenius' equation describes only the temperature dependence of a reaction rate and does not include the entropy changes, so that the activation energy corresponds to the activation enthalpy. Thus, the stress-dependent term, associated with the rate coefficient but not with the enthalpy, should belong either to the entropy term, if it depends on stress, or to another rate coefficient of the probability expression in terms of the free energy, if it does not depend on stress.The analysis of the results leads to the following conclusions: (1) if stress influences entropy, applied stress effectively decreases the activation free energy, and on the other hand the activation enthalpy increases with increasing stress; (2) if the entropy does not depend on stress, applied stress extraordinarily increases the frequency factor that depends on stress. This would mean that the density of active sites is augmented to very high order. Both the activation enthalpy and free energy increase with increasing stress.In both cases, therefore, rate of stress-corrosion cracking increases with applied stress. It can not be made clear, however, on the basis of this kind of analysis, which view is true, and, furthermore, there would be a case in which stress could influence both the entropy and the frequency terms simultaneously. At present, one can only say that the stress-dependent term, associated with thermal excitement of atoms but not with potential energy, is a sort of environment-sensitive parameter that may be able to control the susceptibility to stress-corrosion cracking, although the detailed mechanism involved can not be clarified.
1 0 0 0 防錆技術学校の終業式に臨んで
- 著者
- 藤崎 辰夫
- 出版者
- 社団法人 腐食防食協会
- 雑誌
- 防蝕技術
- 巻号頁・発行日
- vol.10, no.11, pp.517-518, 1961
1 0 0 0 OA 鉄系金属の大気中における腐食機構
- 著者
- C. P. Larrabee 中山 忠行
- 出版者
- Japan Society of Corrosion Engineering
- 雑誌
- 防蝕技術 (ISSN:00109355)
- 巻号頁・発行日
- vol.9, no.1, pp.28-32, 1960-01-25 (Released:2009-11-25)
- 参考文献数
- 14
1 0 0 0 OA 銅および銅合金の変色について
- 著者
- 仲田 進一
- 出版者
- 社団法人 腐食防食協会
- 雑誌
- 防蝕技術 (ISSN:00109355)
- 巻号頁・発行日
- vol.8, no.7, pp.291-297, 1959-07-25 (Released:2009-11-25)
- 参考文献数
- 8
- 被引用文献数
- 1 1
- 著者
- W. L. Williams 伊佐 重輝
- 出版者
- Japan Society of Corrosion Engineering
- 雑誌
- 防蝕技術 (ISSN:00109355)
- 巻号頁・発行日
- vol.7, no.6, pp.320-325, 1958-11-10 (Released:2009-11-25)
- 参考文献数
- 16
1 0 0 0 OA 腐食量の非破壊的測定法
- 著者
- 沢柳 文夫
- 出版者
- 社団法人 腐食防食協会
- 雑誌
- 防蝕技術 (ISSN:00109355)
- 巻号頁・発行日
- vol.10, no.3, pp.105-112,126, 1961-03-15 (Released:2009-11-25)
- 参考文献数
- 46
1 0 0 0 腐食量の非破壊的測定法
- 著者
- 沢柳 文夫
- 出版者
- 社団法人 腐食防食協会
- 雑誌
- 防蝕技術
- 巻号頁・発行日
- vol.10, no.3, pp.105-112,126, 1961
1 0 0 0 化学工業におけるチタンの有用性
- 著者
- 大塚 陸郎 Renshaw W. G. Bish P. R.
- 出版者
- Japan Society of Corrosion Engineering
- 雑誌
- 防蝕技術 (ISSN:00109355)
- 巻号頁・発行日
- vol.4, no.5, pp.210-214,228, 1955
1 0 0 0 防錆技術学校の終業式に臨んで
- 著者
- 藤崎 辰夫
- 出版者
- Japan Society of Corrosion Engineering
- 雑誌
- 防蝕技術 (ISSN:00109355)
- 巻号頁・発行日
- vol.10, no.11, pp.517-518, 1961
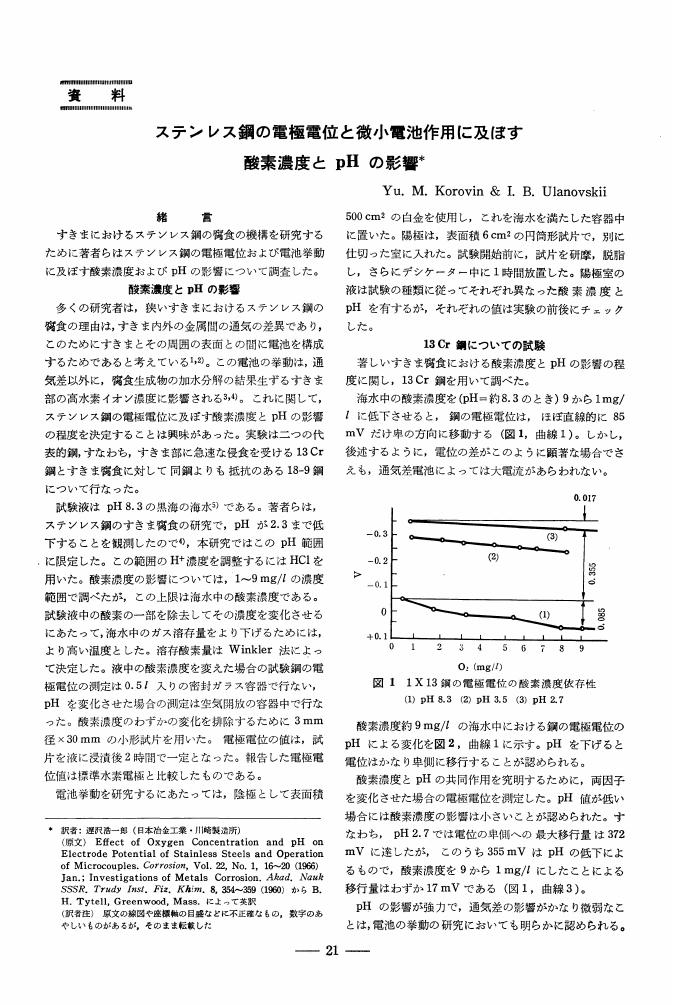
1 0 0 0 OA ステンレス鋼の電極電位と微小電池作用に及ぼす酸素濃度とpHの影響
- 著者
- Yu. M. Korovin I. B. Ulanovskii 遅沢 浩一郎
- 出版者
- Japan Society of Corrosion Engineering
- 雑誌
- 防蝕技術 (ISSN:00109355)
- 巻号頁・発行日
- vol.15, no.7, pp.309-312, 1966-07-15 (Released:2009-11-25)
- 参考文献数
- 6
1 0 0 0 OA 原子炉用金属材料の腐食に及ぼす放射線照射の影響
- 著者
- 沢柳 文夫
- 出版者
- 社団法人 腐食防食協会
- 雑誌
- 防蝕技術 (ISSN:00109355)
- 巻号頁・発行日
- vol.8, no.9, pp.373-380, 1959-09-25 (Released:2009-11-25)
- 参考文献数
- 42
1 0 0 0 OA 沸騰塩化マグネシウム溶液中における応力腐食試験装置
- 著者
- M. A. Streicher A. J. Sweet 石原 只雄
- 出版者
- Japan Society of Corrosion Engineering
- 雑誌
- 防蝕技術 (ISSN:00109355)
- 巻号頁・発行日
- vol.18, no.8, pp.361-366, 1969-08-15 (Released:2009-11-25)
- 参考文献数
- 9
1 0 0 0 OA 米国海軍艦艇の蒸気復水器の性能 (抄訳)
- 著者
- 花田 政明 H. E. Bethon
- 出版者
- Japan Society of Corrosion Engineering
- 雑誌
- 防蝕技術 (ISSN:00109355)
- 巻号頁・発行日
- vol.3, no.3, pp.119-120, 1954-06-20 (Released:2009-11-25)