1 0 0 0 OA リン濃縮スラグからのリンの分離
- 著者
- 岩間 崇之 井上 亮 中瀬 憲治 植田 滋
- 出版者
- 一般社団法人 日本鉄鋼協会
- 雑誌
- 鉄と鋼 (ISSN:00211575)
- 巻号頁・発行日
- vol.109, no.1, pp.1-12, 2023 (Released:2022-12-31)
- 参考文献数
- 31
- 被引用文献数
- 4
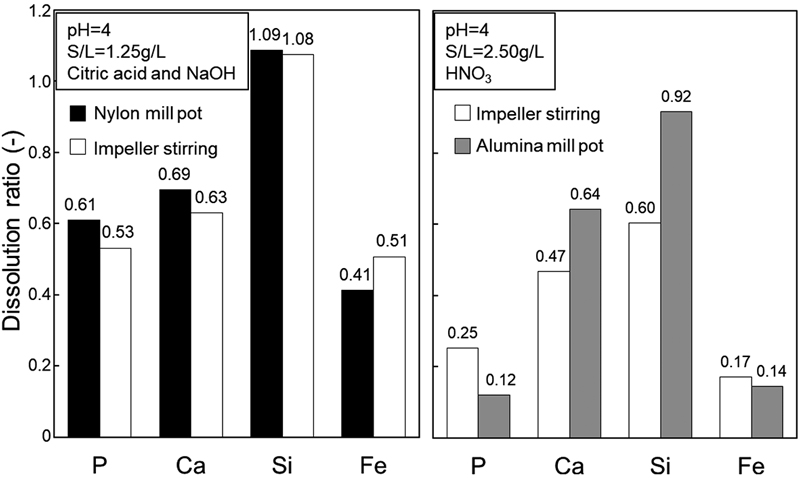
Since 10 million tons of steelmaking slag, which contains a few percent of phosphorus, are annually produced, the phosphorus amount in the slag is equivalent to the annual import volume of phosphorus rock in Japan. Therefore, the steelmaking slag is attracting attention as a potential phosphorus resources. Phosphorus-concentrated slag obtained by the dephosphorization reaction between high phosphorus hot metal and oxidizing slag at high temperature contains phosphorous comparable to that of phosphorus rock. However, because of high FeO concentration, it is difficult to use for phosphorus resources directly. In this work, the effects of pH, acid type and leaching method on the dissolution behavior of phosphorus from P-concentrated slag were investigated. As a result, phosphorus dissolution progressed at lower pH, and was promoted by the addition of citric acid, which is known as a chelate former. When nylon mill pot stirring with citric acid and alumina mill pot stirring with nitric acid were compared to impeller stirring, respectively. By combining nylon mill pot stirring and citrate leachate, phosphorus dissolution was accelerated, because the slag was pulverized during stirring and a formation of insoluble metal-phosphate was inhibited by the formation of complex ion between leached metal cation and citrate. When the slag was leached with alumina mill pot while controlling pH by nitric acid, the phosphorus dissolution ratio lowered since phosphorus ion and aluminum ion, which is supplied by the dissolution of pot and crushing ball during leaching, constructed secondary products with low solubility along with other dissolved ions.