1 0 0 0 Synergistic Effect between Fe4+ and Co4+ on Oxygen Evolution Reaction Catalysis for CaFe1−xCoxO3
- 著者
- Ikuya Yamada Atsushi Tanaka Seiji Oda Yuichi Okazaki Fumito Toda Yuta Kato Yuta Kizawa Masaya Oshita Manami Goto Amane Morimura Asuka Ochi Kaoru Toda Wencong Wang Hajime Yamamoto Hidekazu Ikeno Shunsuke Yagi
- 出版者
- The Japan Institute of Metals and Materials
- 雑誌
- MATERIALS TRANSACTIONS (ISSN:13459678)
- 巻号頁・発行日
- vol.64, no.9, pp.2097-2104, 2023-09-01 (Released:2023-08-25)
- 参考文献数
- 37
Chemical substitution is an effective way to improve electrocatalytic properties in transition metal oxides. We investigate the synergistic effect between Fe4+ and Co4+ ions on the catalytic activity for oxygen evolution reaction (OER) in the Fe–Co-mixed perovskite oxide CaFe1−xCoxO3. The OER activity of CaFe1−xCoxO3 is substantially increased by small amounts of Co (Fe) doping into CaFeO3 (CaCoO3), leading to the superiority compared to the pure Fe and Co perovskite oxides. The x dependences of the OER overpotential and specific activity for CaFe1−xCoxO3 (0.05 ≦ x ≦ 0.95) are expressed by constant offset from the weighted average between CaFeO3 and CaCoO3, which can be interpreted to be the synergistic effect between Fe4+ and Co4+ ions on OER activity. The absence of the optimum x for the highest activity for CaFe1−xCoxO3 contrasts with the volcano-like plots reported in various mixed-metal oxides. First-principle calculations using the special quasirandom structure models on CaFe1−xCoxO3 (x = 0.03–0.5) demonstrate that about half the amount of Fe4+ is electronically activated to possess smaller charge-transfer energies, corroborating the enhancement of catalytic activity in CaFe1−xCoxO3. These findings provide new insight into the synergistic effects in complex transition metal oxide catalysts.
- 著者
- Ikuya Yamada Yuta Kato Hiroshi Nakajima Hidekazu Ikeno Shigeo Mori Shogo Kawaguchi
- 出版者
- The Japan Institute of Metals and Materials
- 雑誌
- MATERIALS TRANSACTIONS (ISSN:13459678)
- 巻号頁・発行日
- pp.MT-MG2022005, (Released:2023-01-10)
- 参考文献数
- 37
- 被引用文献数
- 1
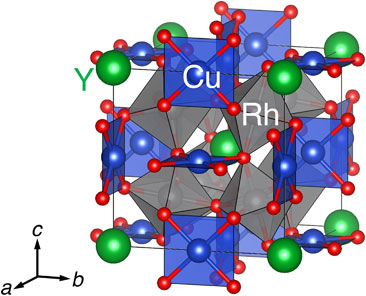
A novel oxide YCu3Rh4O12 has been obtained using high-pressure and high-temperature conditions of 12 GPa and 1573 K. Electron diffraction and synchrotron X-ray powder diffraction data demonstrates that YCu3Rh4O12 crystallizes in a cubic AA′3B4O12-type quadruple perovskite structure. The valence state is estimated to be Y3+Cu3+3Rh3+4O12 by X-ray absorption spectroscopy. The electric resistivity and magnetization data prove that YCu3Rh4O12 is a diamagnetic insulator, which is expected from the electron configurations of Cu3+ (3d8, low spin, S = 0) and Rh3+ (4d6, low spin, S = 0) ions. The first-principle calculation displays the insulating band structure for YCu3Rh4O12. The valence state transition from Ca2+Cu2.8+3Rh3.4+4O12 to Y3+Cu3+3Rh3+4O12 indicates that the doped electrons by the substitution of Y3+ for Ca2+ are not simply injected to Cu and/or Rh ions, realizing unusual charge redistributions consisting of the simultaneous Cu oxidation (Cu2.8+ → Cu3+) and Rh reduction (Rh3.4+ → Rh3+).