- 著者
- Ayumi HASEGAWA Keiji MOCHIDA Narumi OGONUKI Michiko HIROSE Toshiko TOMISHIMA Kimiko INOUE Atsuo OGURA
- 出版者
- The Society for Reproduction and Development
- 雑誌
- Journal of Reproduction and Development (ISSN:09168818)
- 巻号頁・発行日
- vol.63, no.6, pp.539-545, 2017 (Released:2017-12-15)
- 参考文献数
- 29
- 被引用文献数
- 7 14
In embryo transfer experiments in mice, pseudopregnant females as recipients are prepared by sterile mating with vasectomized males. Because only females at the proestrus stage accept males, such females are selected from a stock of animals based on the appearance of their external genital tract. Therefore, the efficiency of preparing pseudopregnant females largely depends on the size of female colonies and the skill of the operators who select females for sterile mating. In this study, we examined whether the efficiency of preparing pseudopregnant females could be improved by applying an estrous cycle synchronization method by progesterone (P4) pretreatment, which significantly enhances the superovulation outcome in mice. We confirmed that after two daily injections of P4 (designated Days 1 and 2) in randomly selected females, the estrous cycles of most females (about 85%) were synchronized at metestrus on Day 3. When P4-treated females were paired with vasectomized males for 4 days (Days 4–8), a vaginal plug was found in 63% (20/32) of the females on Day 7. After the transfer of vitrified-warmed embryos into their oviducts, 52% (73/140) of the embryos successfully developed into offspring, the rate being comparable to that of the conventional embryo transfer procedure. Similarly, 77% (24/31) of females became pregnant by fertile mating with intact males for 3 days, which allowed the scheduled preparation of foster mothers. Thus, our estrous cycle synchronization method may omit the conventional experience-based process of visually observing the vagina to choose females for embryo transfer. Furthermore, it is expected that the size of female stocks for recipients can be reduced to less than 20%, which could be a great advantage for facilities/laboratories undertaking mouse-assisted reproductive technology.
- 著者
- Jinsha LIU Keiji MOCHIDA Ayumi HASEGAWA Kimiko INOUE Atsuo OGURA
- 出版者
- THE SOCIETY FOR REPRODUCTION AND DEVELOPMENT
- 雑誌
- Journal of Reproduction and Development (ISSN:09168818)
- 巻号頁・発行日
- vol.64, no.2, pp.117-127, 2018 (Released:2018-04-13)
- 参考文献数
- 44
- 被引用文献数
- 5
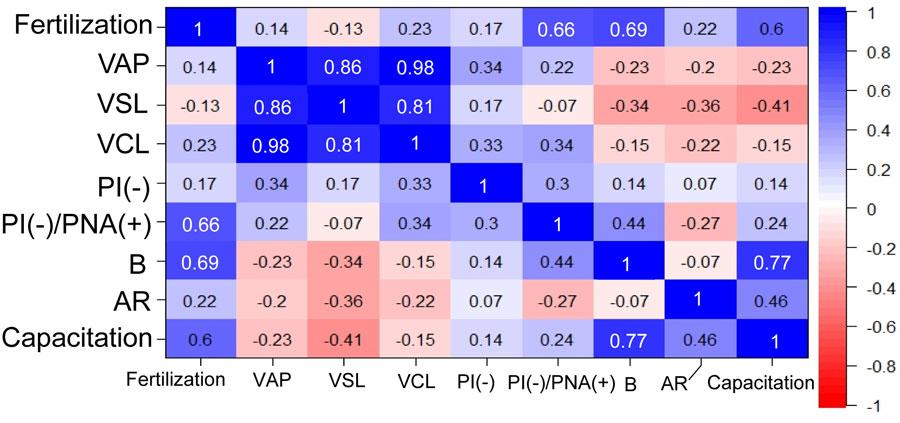
Although it is known that the susceptibility of mouse spermatozoa to freezing-thawing varies greatly with genetic background, the underlying mechanisms remain to be elucidated. In this study, to map genetic regions responsible for the susceptibility of spermatozoa to freezing-thawing, we performed in vitro fertilization using spermatozoa from recombinant inbred mice derived from the C57BL/6J and DBA/2J strains, whose spermatozoa showed distinct fertilization abilities after freezing. Genome-wide interval mapping identified two suggestive quantitative trait loci (QTL) associated with fertilization on chromosomes 1 and 11. The strongest QTL on chromosome 11 included 70 genes at 59.237260–61.324742 Mb and another QTL on chromosome 1 included 43 genes at 153.969506–158.217850 Mb. These regions included at least 15 genes involved with testicular expression and possibly with capacitation or sperm motility. Specifically, the Abl2 gene on chromosome 1, which may affect subcellular actin distribution, had polymorphisms between C57BL/6J and DBA/2J that caused at least three amino acid substitutions. A correlation analysis using recombinant inbred strains revealed that the fertilization rate was strongly correlated with the capacitation rate of frozen-thawed spermatozoa after preincubation. This result is consistent with the fact that C57BL/6J frozen-thawed spermatozoa recover their fertilization capacity following treatment with methyl-β-cyclodextrin to enhance sperm capacitation. Thus, our data provide important clues to the molecular mechanisms underlying cryodamage to mouse spermatozoa.
- 著者
- Jinsha LIU Keiji MOCHIDA Ayumi HASEGAWA Kimiko INOUE Atsuo OGURA
- 出版者
- THE SOCIETY FOR REPRODUCTION AND DEVELOPMENT
- 雑誌
- Journal of Reproduction and Development (ISSN:09168818)
- 巻号頁・発行日
- pp.2017-148, (Released:2017-12-21)
- 被引用文献数
- 5
Although it is known that the susceptibility of mouse spermatozoa to freezing–thawing varies greatly with genetic background, the underlying mechanisms remain to be elucidated. In this study, to map genetic regions responsible for the susceptibility of spermatozoa to freezing–thawing, we performed in vitro fertilization using spermatozoa from recombinant inbred mice derived from the C57BL/6J and DBA/2J strains, whose spermatozoa showed distinct fertilization abilities after freezing. Genome-wide interval mapping identified two suggestive quantitative trait loci (QTL) associated with fertilization on chromosomes 1 and 11. The strongest QTL on chromosome 11 included 70 genes at 59.237260–61.324742 Mb and another QTL on chromosome 1 included 43 genes at 153.969506–158.217850 Mb. These regions included at least 15 genes involved with testicular expression and possibly with capacitation or sperm motility. Specifically, the Abl2 gene on chromosome 1, which may affect subcellular actin distribution, had polymorphisms between C57BL/6J and DBA/2J that caused at least three amino acid substitutions. A correlation analysis using recombinant inbred strains revealed that the fertilization rate was strongly correlated with the capacitation rate of frozen-thawed spermatozoa after preincubation. This result is consistent with the fact that C57BL/6J frozen–thawed spermatozoa recover their fertilization capacity following treatment with methyl-β-cyclodextrin to enhance sperm capacitation. Thus, our data provide important clues to the molecular mechanisms underlying cryodamage to mouse spermatozoa.
- 著者
- Ayumi HASEGAWA Kazuya YONEZAWA Akihiko OHTA Keiji MOCHIDA Atsuo OGURA
- 出版者
- 日本繁殖生物学会
- 雑誌
- Journal of Reproduction and Development (ISSN:09168818)
- 巻号頁・発行日
- vol.58, no.1, pp.156-161, 2012 (Released:2012-03-22)
- 参考文献数
- 30
- 被引用文献数
- 1 18 4
The rapid increase in the number of genetically modified mouse strains has produced a high demand for their frozen spermatozoa from laboratories and mouse banking facilities. Historically, plastic straws have been used preferentially as containers for frozen mammalian spermatozoa because spermatozoa frozen in plastic straws have a high survival rate after thawing. However, plastic straws are more fragile and are used less often than the cryotubes used for conventional cell freezing. In this study, we sought to develop a new protocol for sperm freezing using cryotubes as the container to increase the accessibility of mouse sperm cryopreservation. Epididymal spermatozoa were collected from mature ICR or C57BL/6J (B6) males and were suspended in 18% raffinose and 3% skim milk solution. We then optimized the following conditions using the sperm survival rate as an index: 1) distance of cryotubes from the surface of the liquid nitrogen at freezing, 2) volume of the sperm suspension in the cryotube and 3) temperature of warming sperm during thawing. The best result was obtained when cryotubes containing 10 μl of sperm suspension were immersed 1 cm below the surface of the liquid nitrogen and then thawed at 50 C. The fertilization rates using spermatozoa frozen and thawed using this method were 63.1% in ICR mice and 28.2% in B6 mice. The latter rate was increased to 62.3% by adding reduced glutathione to the fertilization medium. After embryo transfer, 68% and 62% of the fertilized oocytes developed into normal offspring in the ICR and B6 strains, respectively. These results show that cryotubes can be used for cryopreservation of mouse spermatozoa under optimized conditions. This protocol is easy and reproducible, and it may be used in laboratories that do not specialize in sperm cryopreservation.