- 著者
- Kohei Tsuji Takuya Kobayakawa Takahiro Ishii Nobuyo Higashi-Kuwata Chika Azuma Kouki Shinohara Yutaro Miura Kenichi Yamamoto Soshi Nishimura Shin-ichiro Hattori Haydar Bulut Hiroaki Mitsuya Hirokazu Tamamura
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.71, no.12, pp.879-886, 2023-12-01 (Released:2023-12-01)
- 参考文献数
- 53
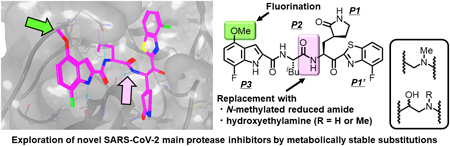
In the development of anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) drugs, its main protease (Mpro), which is an essential enzyme for viral replication, is a promising target. To date, the Mpro inhibitors, nirmatrelvir and ensitrelvir, have been clinically developed by Pfizer Inc. and Shionogi & Co., Ltd., respectively, as orally administrable drugs to treat coronavirus disease of 2019 (COVID-19). We have also developed several potent inhibitors of SARS-CoV-2 Mpro that include compounds 4, 5, TKB245 (6), and TKB248 (7), which possesses a 4-fluorobenzothiazole ketone moiety as a reactive warhead. In compounds 5 and TKB248 (7) we have also found that replacement of the P1-P2 amide of compounds 4 and TKB245 (6) with the corresponding thioamide improved their pharmacokinetics (PK) profile in mice. Here, we report the design, synthesis and evaluation of SARS-CoV-2 Mpro inhibitors with replacement of a digestible amide bond by surrogates (9–11, 33, and 34) and introduction of fluorine atoms in a metabolically reactive methyl group on the indole moiety (8). As the results, these compounds showed comparable or less potency compared to the corresponding parent compounds, YH-53/5h (2) and 4. These results should provide useful information for further development of Mpro inhibitors.