- 著者
- Masamitsu Maekawa Keitaro Miyoshi Aya Narita Toshihiro Sato Yu Sato Masaki Kumondai Masafumi Kikuchi Katsumi Higaki Torayuki Okuyama Yoshikatsu Eto Hiroshi Sakamaki Nariyasu Mano
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.45, no.9, pp.1259-1268, 2022-09-01 (Released:2022-09-01)
- 参考文献数
- 65
- 被引用文献数
- 3
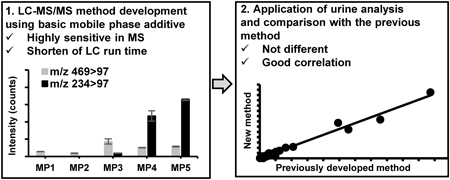
As Niemann–Pick disease type C (NPC) is difficult to diagnose owing to its various clinical symptoms; biomarker tests have been developed. Previously, we revealed urinary sulfated cholesterol metabolites as noninvasive biomarkers for NPC. However, LC/tandem mass spectrometry (LC/MS/MS) requires long separation time and large urine volumes. Recently, a basic mobile phase was reported to increase the MS intensity. Thus, we developed a highly sensitive and rapid LC/MS/MS method for analyzing urinary cholesterol metabolites using a basic mobile phase additive. 3β-Sulfooxy-7β-N-acetylglucosaminyl-5-cholenic acid, its glycine and taurine conjugates, 3β-sulfooxy-7β-hydroxy-5-cholenic acid, and 7-oxo form were measured, with selected reaction monitoring in negative ion mode. Oasis HLB and L-column 3 were used for column-switching LC/MS/MS and urine diluted 10-fold was employed as the sample. After trapping, gradient separation was performed using solutions containing 1% (v/v) ammonium solution. On average, a 16-fold increase in peak areas was observed compared to that obtained at pH 5.5 with the mobile phases. Although the previous method needed 60 min for separation from interference peaks, we succeeded to separate them in 7 min with optimized LC condition. Further, all compounds showed good linearity from 0.3–1000 ng/mL, with satisfactory intra- and inter-day reproducibility. The developed method was applied to the urinalysis of healthy participants and NPC patients. Overall, the concentrations of metabolites correlated with those obtained using the previous method. Therefore, we succeeded to increasing MS intensity and shorten LC running time; and the method is useful for the noninvasive diagnostic screening of patients with NPC.
- 著者
- Masaki Kumondai Masamitsu Maekawa Eiji Hishinuma Yu Sato Toshihiro Sato Masafumi Kikuchi Masahiro Hiratsuka Nariyasu Mano
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.46, no.3, pp.455-463, 2023-03-01 (Released:2023-03-01)
- 参考文献数
- 52
CYP3A4, which contributes to the metabolism of more than 30% of clinically used drugs, exhibits high variation in its activity; therefore, predicting CYP3A4 activity before drug treatment is vital for determining the optimal dosage for each patient. We aimed to develop and validate an LC-tandem mass spectrometry (LC-MS/MS) method that simultaneously measures the levels of CYP3A4 activity-related predictive biomarkers (6β-hydroxycortisol (6β-OHC), cortisol (C), 1β-hydroxydeoxycholic acid (1β-OHDCA), and deoxycholic acid (DCA)). Chromatographic separation was achieved using a YMC-Triart C18 column and a gradient flow of the mobile phase comprising deionized water/25% ammonia solution (100 : 0.1, v/v) and methanol/acetonitrile/25% ammonia solution (50 : 50 : 0.1, v/v/v). Selective reaction monitoring in the negative-ion mode was used for MS/MS, and run times of 33 min were used. All analytes showed high linearity in the range of 3–3000 ng/mL. Additionally, their concentrations in urine samples derived from volunteers were analyzed via treatment with deconjugation enzymes, ignoring inter-individual differences in the variation of other enzymatic activities. Our method satisfied the analytical validation criteria under clinical conditions. Moreover, the concentrations of each analyte were quantified within the range of calibration curves for all urine samples. The conjugated forms of each analyte were hydrolyzed to accurately examine CYP3A4 activity. Non-invasive urine sampling employed herein is an effective alternative to invasive plasma sampling. The analytically validated simultaneous quantification method developed in this study can be used to predict CYP3A4 activity in precision medicine and investigate the potential clinical applications of CYP3A4 biomarkers (6β-OHC/C and 1β-OHDCA/DCA ratios).