- 著者
- Yuhei Tachi Satoru G. Itoh Hisashi Okumura
- 出版者
- The Biophysical Society of Japan
- 雑誌
- Biophysics and Physicobiology (ISSN:21894779)
- 巻号頁・発行日
- vol.19, pp.e190010, 2022 (Released:2022-04-20)
- 参考文献数
- 145
- 被引用文献数
- 3
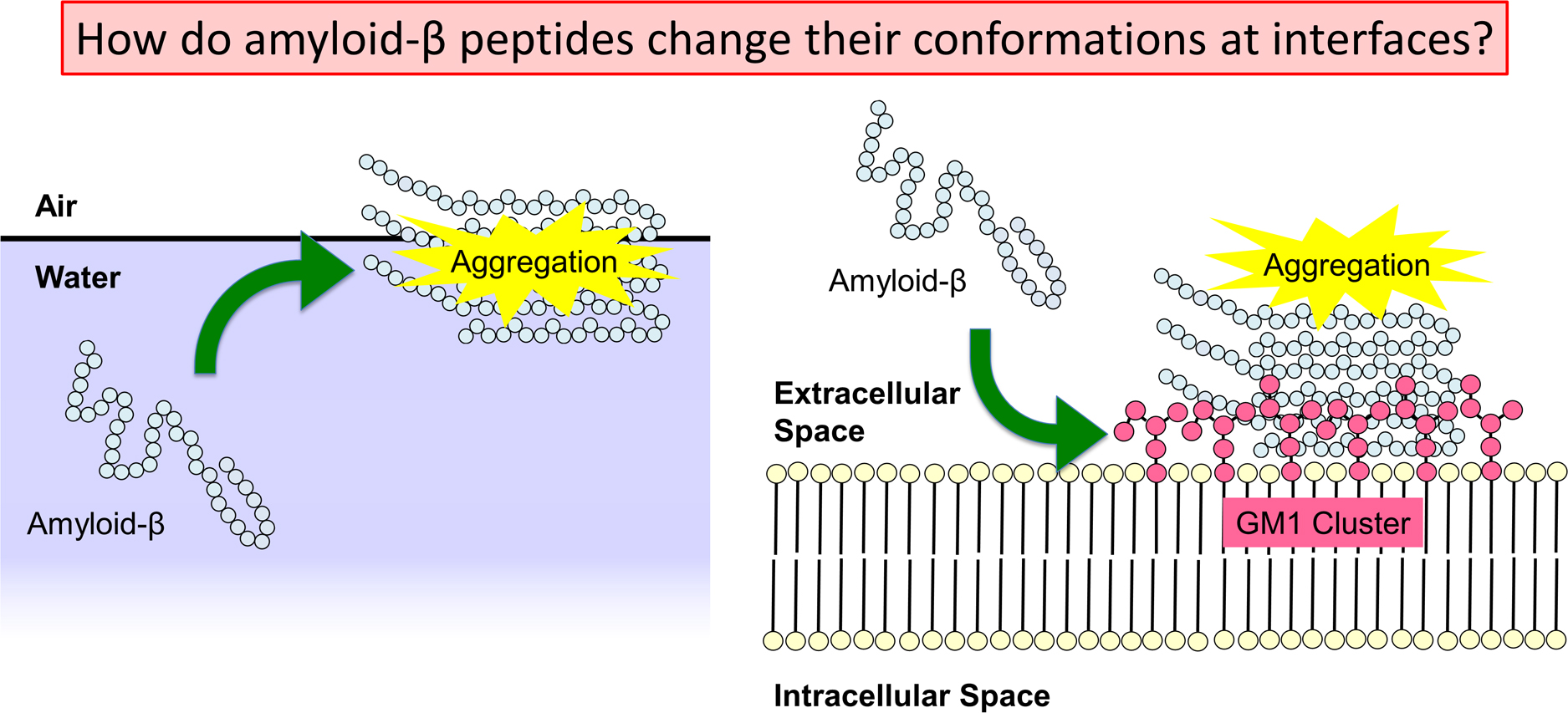
Alzheimer’s disease is thought to be caused by the aggregation of amyloid-β (Aβ) peptides. Their aggregation is accelerated at hydrophilic/hydrophobic interfaces such as the air–water interface and the surface of monosialotetrahexosylganglioside (GM1) clusters on neuronal cell membranes. In this review, we present recent studies of full-length Aβ (Aβ40) peptides and Aβ(16–22) fragments in such heterogeneous environments by molecular dynamics (MD) simulations. These peptides have both hydrophilic and hydrophobic amino-acid residues and tend to exist at the hydrophilic/hydrophobic interface. Therefore, the peptide concentration increases at the interface, which is one of the factors that promote aggregation. Furthermore, it was found that Aβ40 forms an α-helix structure and then a β-hairpin structure at the interface. The β-hairpin promotes the formation of oligomers with intermolecular β-sheets. It means that not only the high concentration of Aβ40 at the interface but also the structure of Aβ40 itself promotes aggregation. In addition, MD simulations of Aβ40 on recently-developed GM1-glycan clusters showed that the HHQ (13–15) segment of Aβ40 is important for the recognition of GM1-glycan clusters. It was also elucidated that Aβ40 forms a helix structure in the C-terminal region on the GM1-glycan cluster. This result suggests that the helix formation, which is the first step in the conformational changes toward pathological aggregation, is initiated at the GM1-glycan moieties rather than at the lipid-ceramide moieties. These studies will enhance the physicochemical understanding of the structural changes of Aβ at the heterogeneous interfaces and the mechanism of Alzheimer’s disease pathogenesis.