- 著者
- Masayoshi Kumai Shungo Imai Shintaro Kato Ryo Koyanagi Kenkichi Tsuruga Takehiro Yamada Yoh Takekuma Mitsuru Sugawara
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.44, no.4, pp.593-598, 2021-04-01 (Released:2021-04-01)
- 参考文献数
- 21
- 被引用文献数
- 4
Nausea is a typical adverse event associated with opioids. In this study, we performed logistic regression analysis with the aim of clarifying the risk factors for nausea induced by extended-release oxycodone (ER-OXY). Furthermore, we constructed a decision tree (DT) model, a typical data mining method, to estimate the risk of oxycodone-induced nausea by combining multiple factors. A retrospective study was conducted on patients who newly received ER-OXY for cancer pain during hospitalization at Hokkaido University Hospital in Japan from April 2015 to March 2018. In logistic regression and DT analyses, the dependent variable was the presence or absence of nausea. Independent variables were the potential risk factors. First, univariate analyses were performed to screen potential factors associated with oxycodone-induced nausea. Then, multivariate and DT analyses were performed using factors with p-values <0.1 in the univariate analysis. Of 267 cases included in this study, nausea was observed in 30.3% (81/267). In multivariate logistic regression analysis, only female sex was extracted as an independent factor affecting nausea (odds ratio, 1.98). In the DT analysis, we additionally revealed that an age <50 years was a risk factor for nausea in female patients. Thus, our DT model indicated that the risk of ER-OXY-induced nausea was highest in the subgroup comprising females <50 years of age (66.7%) and lowest in male patients (25.1%). The DT model suggested that the factor of young women may be an increased risk of ER-OXY-induced nausea.
- 著者
- Takehiro Yamada Shungo Imai Yasuyuki Koshizuka Yuki Tazawa Keisuke Kagami Naoki Tomiyama Ryosuke Sugawara Akira Yamagami Tsuyoshi Shimamura Ken Iseki
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.41, no.7, pp.1112-1118, 2018-07-01 (Released:2018-07-01)
- 参考文献数
- 31
- 被引用文献数
- 15 17
Therapeutic drug monitoring for voriconazole, an antifungal agent, is essential for maximizing efficacy and preventing toxicity. The aim of this study was to elucidate the optimal maintenance dose of voriconazole in patients with severe liver cirrhosis (Child–Pugh class C) by reviewing the plasma trough concentrations obtained by therapeutic drug monitoring and daily doses of voriconazole. We retrospectively evaluated 6 patients with Child–Pugh class C cirrhosis who received oral voriconazole treatment and were liver transplant recipients or were awaiting liver transplantation. We compared their voriconazole trough concentrations and daily maintenance doses to those of patients who did not have liver cirrhosis (n=56). We found that plasma voriconazole trough concentrations in all patients with Child–Pugh class C were almost within therapeutic range, and the median plasma trough concentration at steady state was not significantly different from that of patients who did not have liver cirrhosis. In addition, the median daily maintenance dose of voriconazole was significantly lower (2.13 mg/kg/d) than that of the control patients (6.27 mg/kg/d), suggesting that trough voriconazole concentrations are elevated in Child–Pugh class C patients. Thus, we conclude that oral voriconazole maintenance doses in patients with Child–Pugh class C should be reduced to approximately one-third that of patients with normal liver function, with the follow-up dose adjusted by therapeutic drug monitoring.
- 著者
- Takehiro Yamada Shuhei Ishikawa Nobuhisa Ishiguro Masaki Kobayashi Ken Iseki
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.43, no.9, pp.1338-1345, 2020-09-01 (Released:2020-09-01)
- 参考文献数
- 28
- 被引用文献数
- 2 3
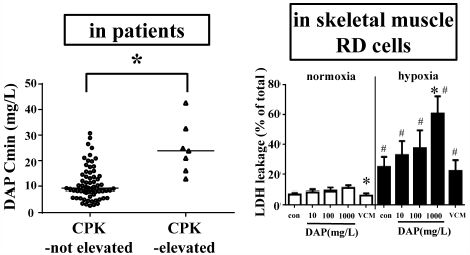
Daptomycin, a cyclic lipopeptide antibiotic, has bactericidal activity against Gram-positive organisms and is especially effective against methicillin-resistant Staphylococcus aureus. Although daptomycin causes unique adverse drug reactions such as elevation of creatine phosphokinase or rhabdomyolysis, the detailed mechanisms underlying these adverse drug reactions in skeletal muscle are unclear. This study aimed to elucidate whether daptomycin causes direct skeletal muscle cell toxicity and investigate the relationship between daptomycin exposure and musculoskeletal toxicity. First, we evaluated the relationship between daptomycin exposure and skeletal muscle toxicity. Of the 38 patients who received daptomycin intravenously, an elevation in creatine phosphokinase levels was observed in five. The median plasma trough concentration of daptomycin in patients with elevated creatine phosphokinase levels was significantly higher than that in patients whose creatine phosphokinase levels were within the normal range, suggesting that increased exposure to daptomycin is related to elevation in creatine phosphokinase levels. In an in vitro study using human rhabdomyosarcoma cells, daptomycin reduced cell viability and increased membrane damage. These effects were more marked under hypoxic conditions. A necroptotic pathway seemed to be involved because phosphorylated mixed lineage kinase domain-like protein expression was enhanced following daptomycin exposure, which was significantly enhanced under hypoxic conditions. These findings indicate that daptomycin elicits cytotoxic effects against skeletal muscle cells via the necroptotic pathway, and the extent of toxicity is enhanced under hypoxic conditions.