- 著者
- Toshinori Hirai Hidefumi Kasai Masahiro Takahashi Satomi Uchida Naoko Akai Kazuhiko Hanada Toshimasa Itoh Takuya Iwamoto
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.45, no.7, pp.948-954, 2022-07-01 (Released:2022-07-01)
- 参考文献数
- 35
Some population pharmacokinetic models for amiodarone (AMD) did not incorporate N-desethylamiodarone (DEA) concentration. Glucocorticoids activate CYP3A4 activity, metabolizing AMD. In contrast, CYP3A4 activity may decrease under inflammation conditions. However, direct evidence for the role of glucocorticoid or inflammation on the pharmacokinetics of AMD and DEA is lacking. The pilot study aimed to address this gap using a population pharmacokinetic analysis of AMD and DEA. A retrospective cohort observational study in adult patients who underwent AMD treatment with trough concentration measurement was conducted at Tokyo Women’s Medical University, Medical Center East from June 2015 to March 2019. Both structural models of AMD and DEA applied 1-compartment models, which included significant covariates using a stepwise forward selection and backward elimination method. The eligible 81 patients (C-reactive protein level: 0.26 [interquartile range; 0.09–1.92] mg/dL) had a total of 408 trough concentrations for both AMD and DEA. The median trough concentrations were 0.49 [0.31–0.81] µg/mL for AMD and 0.43 [0.28–0.71] µg/mL for DEA during a median follow-up period of 446 [147–1059] d. Three patients received low-dose oral glucocorticoid. The final model identified that AMD clearance was 7.9 L/h, and the apparent DEA clearance was 10.3 L/h. Co-administered glucocorticoids lowered apparent DEA clearance by 35%. These results indicate that co-administered glucocorticoids may increase DEA concentrations in patients without severe inflammation.
- 著者
- Toshinori Hirai Chihiro Shiraishi Tomohiro Murata Takuya Iwamoto
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- BPB Reports (ISSN:2434432X)
- 巻号頁・発行日
- vol.4, no.5, pp.170-174, 2021 (Released:2021-10-28)
- 参考文献数
- 24
The impact of renal impairment on the drug-drug interaction between azathioprine and allopurinol that causes myelosuppression and hepatotoxicity remains unclear. This case series study investigated adverse effects caused by azathioprine owing to drug-drug interaction considering renal impairment. Patients who started the combination therapy of azathioprine and allopurinol at Mie University Hospital between January 2013 and February 2021 were enrolled. The outcome of adverse events associated with azathioprine was assessed according to Common Terminology Criteria for Adverse Events version 5.0. The Drug Interaction Probability Scale was used to determine the probability of drug-drug interaction. Of the three patients, two were identified as exhibiting drug-drug interaction with the Drug Interaction Probability Scale > 5 points. They experienced grade 3 myelosuppression or hepatotoxicity with fatigue, after initiation of azathioprine (1.28 and 0.44 mg/kg once daily) and allopurinol (50 mg once daily). They received appropriate dose-adjusted allopurinol according to renal function. Additionally, both patients had the estimated glomerular filtration rate < 60 mL/min/1.73 m2. Thus, renal impairment might reduce the excretion of oxypurinol, an active metabolite of allopurinol, which certainly enhances the side effects of azathioprine.
- 著者
- Takahiko Aoyama Toshinori Hirai Yasuhiro Tsuji Aoi Miyamoto Toshimasa Itoh Takuya Iwamoto Yoshiaki Matsumoto
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.45, no.1, pp.136-142, 2022-01-01 (Released:2022-01-01)
- 参考文献数
- 31
- 被引用文献数
- 1
Warfarin is a representative anticoagulant with large interindividual variability. The published kinetic-pharmacodynamic (K-PD) model allows the prediction of warfarin dose requirement in Swedish patients; however, its applicability in Japanese patients is not known. We evaluated the model’s predictive performance in Japanese patients with various backgrounds and relationships using Bayesian parameter estimation and sampling times. A single-center retrospective observational study was conducted at Tokyo Women’s Medical University, Medical Center East. The study population consisted of adult patients aged >20 years who commenced warfarin with a prothrombin time-international normalized ratio (PT-INR) from June 2015 to June 2019. The published K-PD model modified by Wright and Duffull was assessed using prediction-corrected visual predictive checks, focusing on clinical characteristics, including age, renal function, and individual prediction error. The external dataset included 232 patients who received an initial warfarin daily dose of 3.2 ± 1.28 mg with 2278 PT-INR points (median [range] follow-up period of 23 d [7–28]). Prediction-corrected visual predictive checks carried a propensity for underprediction. Additionally, age >60 years, body mass index ≤25 kg/m2, and estimated glomerular filtration rate ≤60 mL/min/1.73 m2 had a pronounced tendency to underpredict PT-INR. However, Bayesian prediction using four prior observations reduced underprediction. To improve the prediction performance of these special populations, further studies are required to construct a model to predict warfarin dose requirements in Japanese patients.
- 著者
- Iqbal Julian Takuya Iwamoto
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.44, no.8, pp.1050-1059, 2021-08-01 (Released:2021-08-01)
- 参考文献数
- 40
- 被引用文献数
- 1
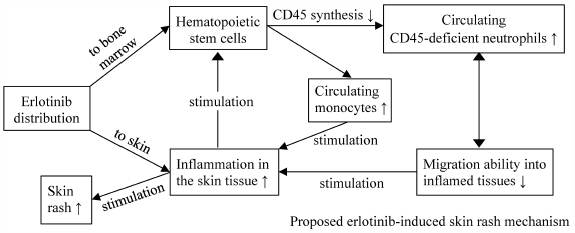
Skin rash is a common adverse event associated with erlotinib therapy. In severe conditions, the rash could affect patients’ QOL. If the rash occurrence can be predicted, erlotinib treatment failures can be prevented. We designed an in vivo study that applied erlotinib regimens resembling its clinical application to evaluate possible erlotinib-induced skin rash biomarkers for humans and simultaneously observe the effects of erlotinib discontinuation, followed with or without dose reduction, on rash development. Rats were divided into four groups: placebo, constant (erlotinib 35 mg/kg on d1–d21), intermittent (erlotinib 70 mg/kg on d1–d7 and d15–d21), and mimic (erlotinib 70 mg/kg on d1–d7 and erlotinib 35 mg/kg on d15–d21). Blood sampling was performed on d1, d8, d15, and d22. The samples were used to measure erlotinib concentrations, the level of hepatic and renal function markers, immune cell percentages, and immune cells’ CD45 expression levels. Erlotinib 70 mg/kg generated high mean circulating erlotinib concentrations (>1800 ng/mL) that led to severe rashes. Erlotinib dose reduction following rash occurrence reduced circulating erlotinib concentration and rash severity. After the treatment, the escalation of neutrophil percentages and reduction of neutrophils’ CD45 expression levels were observed, which were significantly correlated with the rash occurrence. This study is the first to show that erlotinib-induced skin rash may be affected by the reduction of neutrophils’ CD45 expression levels, and this is a valuable finding to elucidate the erlotinib-induced skin rash formation mechanism.