- 著者
- Taisuke Konno Hiroyuki Suzuki Hitoshi Nakamura Yuriko Murai
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- pp.b22-00050, (Released:2022-06-29)
- 参考文献数
- 15
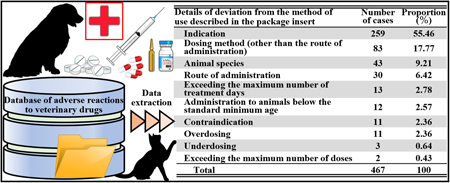
In veterinary medicine, various drugs are used on a daily basis. Using inappropriate medications poses health hazards to companion animals and humans; thus, assessing adverse events in veterinary medicine has great social significance but remains an untapped area of research. In this study, to promote the appropriate use of veterinary drugs and clarify common pharmaceutical issues in Japanese veterinary medicine, we analyzed information in the Veterinary Drug Side Effects Database (National Veterinary Assay Laboratory of the Ministry of Agriculture, Forestry and Fisheries, Japan). We found that the number of reports has been increasing annually, including those on high-risk drugs, molecular-targeted drugs, and antibody-based drugs. The details of the reports were similar to those from the United States, including the misadministration of veterinary drugs to humans, improper drug management, and re-administering drugs with a history of side effects. Furthermore, 46.50% of all reports mentioned the administration of one or more drugs, with the highest number of concomitant drugs being 10. In addition, 37.78% of all reports described the use of drugs in manners deviating from the intended use indicated in the package insert. Therefore, to avoid adverse events, pharmacists may have to be involved in dispensing and aseptically preparing veterinary medicines and providing drug information and medication guidance. To optimize pharmacotherapy for ill companion animals, "veterinary pharmacy" and "veterinary medicine pharmacy" must be developed in line with clinical situations in Japan, while considering knowledge from countries that are advanced in terms of veterinary medicine.
- 著者
- Taisuke Konno Hiroyuki Suzuki Hitoshi Nakamura Yuriko Murai
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.45, no.9, pp.1225-1231, 2022-09-01 (Released:2022-09-01)
- 参考文献数
- 15
In veterinary medicine, various drugs are used on a daily basis. Using inappropriate medications poses health hazards to companion animals and humans; thus, assessing adverse events in veterinary medicine has great social significance but remains an untapped area of research. In this study, to promote the appropriate use of veterinary drugs and clarify common pharmaceutical issues in Japanese veterinary medicine, we analyzed information in the Veterinary Drug Side Effects Database (National Veterinary Assay Laboratory of the Ministry of Agriculture, Forestry and Fisheries, Japan). We found that the number of reports has been increasing annually, including those on high-risk drugs, molecular-targeted drugs, and antibody-based drugs. The details of the reports were similar to those from the United States, including the misadministration of veterinary drugs to humans, improper drug management, and re-administering drugs with a history of side effects. Furthermore, 46.50% of all reports mentioned the administration of one or more drugs, with the highest number of concomitant drugs being 10. In addition, 37.78% of all reports described the use of drugs in manners deviating from the intended use indicated in the package insert. Therefore, to avoid adverse events, pharmacists may have to be involved in dispensing and aseptically preparing veterinary medicines and providing drug information and medication guidance. To optimize pharmacotherapy for ill companion animals, “veterinary pharmacy” and “veterinary medicine pharmacy” must be developed in line with clinical situations in Japan, while considering knowledge from countries that are advanced in terms of veterinary medicine.