- 著者
- Mariko Kawana Akime Miyasato Miyui Funato Keigo Nagatani Norifumi Suzuki Chiharu Onoda Hidenori Fujimoto Rintaro Ohno Ayuko Kusakabe Mio Kiribayashi Kazuyo Nakamura Masayoshi Kondo Ayumi Ozeki Kousuke Okamoto Hideya Kokubun
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.46, no.10, pp.1444-1450, 2023-10-01 (Released:2023-10-01)
- 参考文献数
- 13
In Japan, a low-dose transdermal fentanyl (TDF; 0.5 mg) has been approved to address pain in opioid-naïve patients with cancer; however, efficacy and safety data are lacking. To determine the efficacy and safety of TDF, patients with opioid-naïve cancer pain prescribed TDF (0.5 mg/d) and oral oxycodone sustained-release formulation (OXY) 10 mg/d were extracted from electronic medical and nursing records. Overall, 40 and 101 subjects were analyzed in the TDF and OXY groups, respectively. Compared with baseline (median [minimum, maximum]) values, changes in the Numerical Rating Scale (NRS) score on days 1, 3, and 7 post-administration were as follows: TDF (0 [−5, 4]) and OXY (−1.0 [−8, 3]); TDF (−1.5 [−6, 3]) and OXY (−2.0 [−8, 4]); and TDF (−2.0[−6, 3]) and OXY (−3.0[−8, 5]), respectively. No significant difference was observed between the groups on days 1 and 3; however, the change in the NRS on day 7 was significantly higher in the OXY group than that in the TDF group. Regarding adverse events, nausea occurred in 12.5 and 13.9% of patients in the TDF and OXY groups, respectively, while 12.5% of TDF- and 10.9% of OXY-treated patients experienced somnolence, revealing similar occurrence in both groups. However, constipation was more common in the OXY group (TDF: 50.0%, OXY: 71.3%). No serious adverse events (e.g., respiratory depression) were observed in either group. Low-dose TDF (0.5 mg), available only in Japan, showed comparable efficacy and safety to OXY (10 mg/d) and can be a first choice for opioid-naïve patients with cancer pain.
- 著者
- Shinya Suzuki Mayako Uchida Yukio Suga Hideki Sugawara Hideya Kokubun Yoshihiro Uesawa Takayuki Nakagawa Hisamitsu Takase
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.42, no.7, pp.1164-1171, 2019-07-01 (Released:2019-07-01)
- 参考文献数
- 40
- 被引用文献数
- 2 12
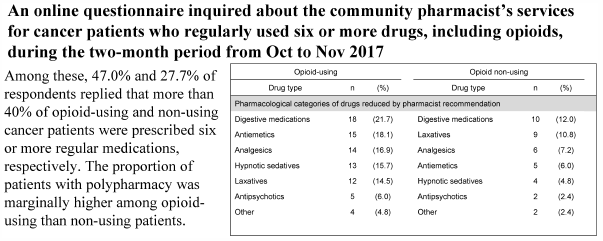
No nationwide study on polypharmacy in palliative care among Japanese community pharmacies has yet been conducted. We conducted an online questionnaire survey for community pharmacist members of The Japanese Society for Pharmaceutical Palliative Care and Sciences regarding their contributions to cancer patients who regularly used six or more drugs, including opioids, in service during the two-month period from October to November 2017. Of 579 community pharmacists, 83 responded to the survey (14.3%). Among them, 47.0 and 27.7% of respondents replied that more than 40% of opioid-using and non-using cancer patients were prescribed six or more regular medications, respectively. The proportion of patients with polypharmacy was marginally higher among opioid-using than non-using patients. Additionally, 31.3 and 22.9% of respondents replied that a low or moderate rate of opioid-using and non-using patients with polypharmacy received inappropriate prescriptions, respectively, including “unnecessary medications,” “adverse drug reactions” and “duplication of pharmacological effect.” The proportion of patients who received inappropriate prescriptions was significantly higher among opioid-using than non-using patients. Furthermore, 37.3 and 19.3% of respondents replied that pharmacist’s recommendations contributed to drug reduction in opioid-using and non-using patients with polypharmacy who received inappropriate prescriptions, respectively. The responders with higher confidence in palliative care showed more success rate for reducing inappropriate medications. Our findings suggest that opioid use can be associated with an increased risk of polypharmacy in cancer patients, and that recommendations by a population of community pharmacists can reduce inappropriate medications and improve adverse drug reactions in both opioid-using and non-using cancer patients with polypharmacy.
- 著者
- Tetsusuke Yoshimoto Emi Ryu Shiro Tomiyasu Minoru Hojo Hideya Kokubun Motohiro Matoba
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.41, no.6, pp.850-857, 2018-06-01 (Released:2018-06-01)
- 参考文献数
- 42
- 被引用文献数
- 4
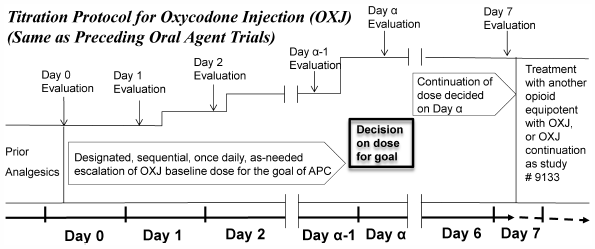
Pure oxycodone injection became increasingly necessary after oral oxycodone was launched in Japan in 2003. However, trials clarifying the efficacy and safety of injection are rare. Therefore, a multicenter open study on injection was designed and carried out in 2010, resulting in the launch of injection therapy in 2012. As published domestic case reports on efficacy already show widespread prescription, this study aimed to provide useful information for cancer pain relief in Japan and other countries. Our oxycodone injection study consisted of two trials, one of intravenous (S#9131) and the other of subcutaneous (S#9132) administration. The minimum required number of enrolled patients suffering cancer pain was determined to be 70 in S#9131 and 20 in S#9132. These studies had the same dose-titration protocol as the main endpoint, i.e., pain relief rate (PRR) defined as the rate of achieving adequate pain control (APC), as in prior oral oxycodone trials in Japan. In S#9131, PRR was 81.4% (95% confidence interval: 70.3–89.7%), therefore, the null hypothesis of PRR<70% was rejected using the binominal one-sided test (p=0.0217). In S#9132, PRR was 73.7% also surpassing 70%. Safety was also assessed in the same way as in prior trials. The majority of adverse effects were moderate or mild and recovered with no sequelae. As shown above, the injection was considered to be effective and safe in cancer pain treatment. The details of these trials, particularly the dose-titration protocol for achieving APC and route switching information, are expected to enhance injection convenience for prescribers.