- 著者
- Yukio Suga Mayako Uchida Shinya Suzuki Hideki Sugawara Kazuhiro Torigoe Akihiko Futamura Yoshihiro Uesawa Takayuki Nakagawa Hisamitsu Takase
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.42, no.5, pp.801-806, 2019-05-01 (Released:2019-05-01)
- 参考文献数
- 24
- 被引用文献数
- 10
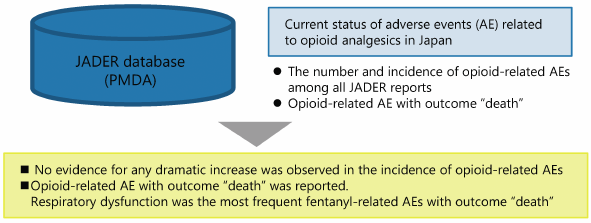
Opioid analgesics have greatly contributed to the advancement of pain management. However, although opioids have been appropriately used in Japan, they rarely induce serious adverse events, such as respiratory depression. The present study aimed to investigate the temporal changes in the occurrence of opioid-related adverse events and deaths between 2004 and 2017 in Japan using the Japanese Adverse Drug Event Report (JADER) database. We analyzed the following points using data extracted from JADER website: 1) temporal changes in the number and proportion of opioid-related adverse event reports; 2) temporal changes in the number of morphine-, oxycodone-, and fentanyl-related adverse event reports per annual consumption; and 3) cases in which the reported outcome following opioid-related adverse events was death. Our results showed no dramatic changes in the overall incidence of opioid-related adverse events, despite the temporal changes in the annual consumption and shared component of each opioid during the survey period. However, the number and rate of fentanyl-related adverse events and their outcome “death” increased since 2010, being the highest among all adverse event including those related to morphine and oxycodone. Outcome “death” by fentanyl-related adverse events was caused mainly due to respiratory depression. These findings suggest that, although opioid-related adverse events can be controlled through proper monitoring and management by medical personnel in Japan, extra caution should be continuously paid for the rare but serious fentanyl-induced adverse events.
- 著者
- Hideyuki Terazono Masami Tsuchiya Yosuke Maki Naoki Yoshikawa Yosuke Kawahara Keiko Nishimura Keisuke Shinohara Daisuke Ogawa Riho Mori Yoshihiro Iwamoto Fumio Itagaki Hiroyuki Masuko Masahito Yonemura Mayako Uchida
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.45, no.7, pp.856-862, 2022-07-01 (Released:2022-07-01)
- 参考文献数
- 19
- 被引用文献数
- 1
It is essential for oncology pharmacists to update their knowledge, skills, and ethical attitudes. The Japanese Society of Pharmaceutical Oncology is an academic society for healthcare professionals involved in cancer treatment. It has conducted in-person seminars every year to cultivate the knowledge necessary for practicing advanced cancer medicine. Owing to the coronavirus disease (COVID-19) pandemic, the society was obligated to conduct a web-based seminar this year. A questionnaire survey was conducted before and after the webinar to explain how it works and to assess the learning attitudes of beginner and moderately skilled pharmacists in the field of oncology. Questionnaire surveys were conducted with the participants before and after watching the webinar. The questionnaires sought to determine participants’ perspectives on the webinar and their knowledge of the seven modules. Of the 1756 webinar attendees, 1661 (94.6%) answered the pre-webinar survey and 1586 (90.3%) answered the post-webinar survey. Results indicate that the median post-webinar knowledge score was significantly higher than the median pre-webinar score (p < 0.001) in all modules. Principal component analysis of the degree of knowledge of seven modules revealed that the improved score group consisted of those from younger age groups, with less experience as pharmacists, non-society members, and those with less experience in past society seminars. Moreover, the web-based seminar provided a uniform learning effect throughout the country without distinguishing between urban and rural learners. The web-based educational program was an acceptable educational tool for Japanese oncology pharmacists.
- 著者
- Shinya Suzuki Mayako Uchida Yukio Suga Hideki Sugawara Hideya Kokubun Yoshihiro Uesawa Takayuki Nakagawa Hisamitsu Takase
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.42, no.7, pp.1164-1171, 2019-07-01 (Released:2019-07-01)
- 参考文献数
- 40
- 被引用文献数
- 2 12
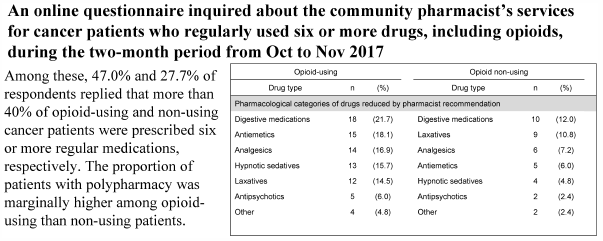
No nationwide study on polypharmacy in palliative care among Japanese community pharmacies has yet been conducted. We conducted an online questionnaire survey for community pharmacist members of The Japanese Society for Pharmaceutical Palliative Care and Sciences regarding their contributions to cancer patients who regularly used six or more drugs, including opioids, in service during the two-month period from October to November 2017. Of 579 community pharmacists, 83 responded to the survey (14.3%). Among them, 47.0 and 27.7% of respondents replied that more than 40% of opioid-using and non-using cancer patients were prescribed six or more regular medications, respectively. The proportion of patients with polypharmacy was marginally higher among opioid-using than non-using patients. Additionally, 31.3 and 22.9% of respondents replied that a low or moderate rate of opioid-using and non-using patients with polypharmacy received inappropriate prescriptions, respectively, including “unnecessary medications,” “adverse drug reactions” and “duplication of pharmacological effect.” The proportion of patients who received inappropriate prescriptions was significantly higher among opioid-using than non-using patients. Furthermore, 37.3 and 19.3% of respondents replied that pharmacist’s recommendations contributed to drug reduction in opioid-using and non-using patients with polypharmacy who received inappropriate prescriptions, respectively. The responders with higher confidence in palliative care showed more success rate for reducing inappropriate medications. Our findings suggest that opioid use can be associated with an increased risk of polypharmacy in cancer patients, and that recommendations by a population of community pharmacists can reduce inappropriate medications and improve adverse drug reactions in both opioid-using and non-using cancer patients with polypharmacy.
- 著者
- Mayako Uchida Yasuhiro Mori Kenta Akiba Moena Miyasaka Tatsuya Hirano Hiroaki Ikesue Yuki Yamaguchi Aoi Takano Nami Maegawa Yoshimitsu Shimomura Keiko Hosohata Nobuyuki Muroi Takayuki Ishikawa Tohru Hashida Tsutomu Nakamura
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.43, no.10, pp.1577-1582, 2020-10-01 (Released:2020-10-01)
- 参考文献数
- 26
- 被引用文献数
- 2
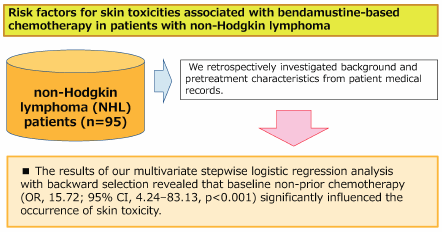
Bendamustine plays an especially important role as a treatment for non-Hodgkin lymphoma (NHL). However, patients administered bendamustine alone or in combination with rituximab (BR) may experience drug-associated skin toxicities that can profoundly impact their health-related QOL through both physical discomfort and psychological distress. Moreover, worsening skin symptoms may lead to dose reduction or termination in the management of cancer chemotherapy. We retrospectively investigated patient backgrounds and pretreatment characteristics from medical records of NHL patients treated with bendamustine alone or BR therapy and identified predictive factors for skin toxicities at the start of chemotherapy. Patients were eligible for the study if they were 20 years older, diagnosed with NHL, and received bendamustine alone or BR therapy at the Department of Hematology, Kobe City Medical Center General Hospital, between April 1, 2011, and March 31, 2018. This study included 95 patients with newly diagnosed or refractory or relapsed NHL. Multivariate stepwise logistic regression analysis with backward selection revealed that baseline non-prior chemotherapy (odds ratio (OR), 15.72; 95% confidence interval (CI), 4.24–83.13, p < 0.001) was a significant factor influencing the occurrence of skin toxicity. Our results demonstrated that non-prior chemotherapy was a significant risk factor for skin toxicities in patients with NHL receiving bendamustine alone or BR therapy. No patient experience serious side effects of grade 3 or higher and that bendamustine is very useful as a first-line treatment.
- 著者
- Mayako Uchida Yuki Yamaguchi Syuhei Hosomi Hiroaki Ikesue Yasuhiro Mori Nami Maegawa Aoi Takano Yuki Sato Keiko Hosohata Nobuyuki Muroi Keisuke Tomii Tohru Hashida Tsutomu Nakamura
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.43, no.8, pp.1235-1240, 2020-08-01 (Released:2020-08-01)
- 参考文献数
- 42
- 被引用文献数
- 3
We retrospectively obtained data of patient background and pretreatment characteristics from medical records and identified the predictive factors of febrile neutropenia (FN) in patients with non-small cell lung cancer (NSCLC) treated with docetaxel alone or in combination with the anti-vascular endothelial growth factor (VEGF) antibody bevacizumab. Patients were eligible for inclusion in the study if they were 20 years or older, diagnosed with NSCLC, and received docetaxel monotherapy alone or in combination with bevacizumab at the Department of Respiratory Medicine, Kobe City Medical Center General Hospital, between July 1, 2011, and March 31, 2018. Eighty-one patients with recurrent or advanced NSCLC were included. Multivariate stepwise logistic regression analysis with backward selection revealed that lower baseline Eastern Cooperative Oncology Group performance status (ECOG-PS) scores of 1 and 2 (odds ratio (OR), 5.098; 95% confidence interval (CI), 1.045–24.879, p = 0.021) and baseline platelet count below 18.8 × 104/µL (OR, 3.861; 95% CI, 1.211–12.311, p = 0.022) were significant factors influencing the FN occurrence rate. Our results demonstrated that ECOG-PS 1–2 and lower baseline platelet count were significant risk factors of FN in patients with NSCLC receiving docetaxel-based chemotherapy. Moreover, the combination of anti-VEGF antibodies and docetaxel might be associated with increased FN frequency. Despite the limitations of this study including its retrospective design, single-center site, and small sample size, baseline ECOG-PS score and platelet count may be regarded as important indices to identify patients for prophylactic granulocyte-colony stimulating factor (G-CSF) treatment before docetaxel-based chemotherapy.