- 著者
- Jichen Wang Hideyuki Suzuki Nanako Nakashima Mariko Kitajima Hiromitsu Takayama Kazuki Saito Mami Yamazaki Naoko Yoshimoto
- 出版者
- Japanese Society for Plant Biotechnology
- 雑誌
- Plant Biotechnology (ISSN:13424580)
- 巻号頁・発行日
- vol.39, no.3, pp.281-289, 2022-09-25 (Released:2022-09-25)
- 参考文献数
- 26
Marasmin [S-(methylthiomethyl)-L-cysteine-4-oxide] is a pharmaceutically valuable sulfur-containing compound produced by the traditional medicinal plant, Tulbaghia violacea. Here, we report the identification of an S-oxygenase, TvMAS1, that produces marasmin from its corresponding sulfide, S-(methylthiomethyl)-L-cysteine. The amino acid sequence of TvMAS1 showed high sequence similarity to known flavin-containing S-oxygenating monooxygenases in plants. Recombinant TvMAS1 catalyzed regiospecific S-oxygenation at S4 of S-(methylthiomethyl)-L-cysteine to yield marasmin, with an apparent Km value of 0.55 mM. TvMAS1 mRNA accumulated with S-(methylthiomethyl)-L-cysteine and marasmin in various organs of T. violacea. Our findings suggest that TvMAS1 catalyzes the S-oxygenation reaction during the last step of marasmin biosynthesis in T. violacea.
- 著者
- Hiromitsu Takayama
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.68, no.2, pp.103-116, 2020-02-01 (Released:2020-02-01)
- 参考文献数
- 67
- 被引用文献数
- 8
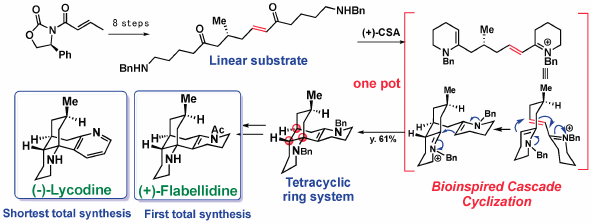
The merits of biogenetic considerations in the chemical syntheses of natural products have been emphasized by describing the total syntheses of Lycopodium alkaloids; lycodine, flabellidine, lycopodine, and flabelliformine, as well as monoterpenoid indole alkaloids; C-mavacurine, kopsiyunnanine K, koumine, and 11-methoxy-19R-hydroxygelselegine.
- 著者
- Hiromitsu Takayama
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.52, no.8, pp.916-928, 2004 (Released:2004-08-10)
- 参考文献数
- 63
- 被引用文献数
- 127 250
The leaves of a tropical plant, Mitragyna speciosa KORTH (Rubiaceae), have been traditionally used as a substitute for opium. Phytochemical studies of the constituents of the plant growing in Thailand and Malaysia have led to the isolation of several 9-methoxy-Corynanthe-type monoterpenoid indole alkaloids, including new natural products. The structures of the new compounds were elucidated by spectroscopic and/or synthetic methods. The potent opioid agonistic activities of mitragynine, the major constituent of this plant, and its analogues were found in in vitro and in vivo experiments and the mechanisms underlying the analgesic activity were clarified. The essential structural features of mitragynines, which differ from those of morphine and are responsible for the analgesic activity, were elucidated by pharmacological evaluation of the natural and synthetic derivatives. Among the mitragynine derivatives, 7-hydroxymitragynine, a minor constituent of M. speciosa, was found to exhibit potent antinociceptive activity in mice.