- 著者
- Jichen Wang Hideyuki Suzuki Nanako Nakashima Mariko Kitajima Hiromitsu Takayama Kazuki Saito Mami Yamazaki Naoko Yoshimoto
- 出版者
- Japanese Society for Plant Biotechnology
- 雑誌
- Plant Biotechnology (ISSN:13424580)
- 巻号頁・発行日
- vol.39, no.3, pp.281-289, 2022-09-25 (Released:2022-09-25)
- 参考文献数
- 26
Marasmin [S-(methylthiomethyl)-L-cysteine-4-oxide] is a pharmaceutically valuable sulfur-containing compound produced by the traditional medicinal plant, Tulbaghia violacea. Here, we report the identification of an S-oxygenase, TvMAS1, that produces marasmin from its corresponding sulfide, S-(methylthiomethyl)-L-cysteine. The amino acid sequence of TvMAS1 showed high sequence similarity to known flavin-containing S-oxygenating monooxygenases in plants. Recombinant TvMAS1 catalyzed regiospecific S-oxygenation at S4 of S-(methylthiomethyl)-L-cysteine to yield marasmin, with an apparent Km value of 0.55 mM. TvMAS1 mRNA accumulated with S-(methylthiomethyl)-L-cysteine and marasmin in various organs of T. violacea. Our findings suggest that TvMAS1 catalyzes the S-oxygenation reaction during the last step of marasmin biosynthesis in T. violacea.
- 著者
- Nanako Nakashima Jukiya Sakamoto Kenta Rakumitsu Mariko Kitajima Lia Dewi Juliawaty Hayato Ishikawa
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.70, no.2, pp.187-191, 2022-02-01 (Released:2022-02-01)
- 参考文献数
- 23
- 被引用文献数
- 5
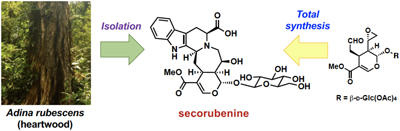
A new pentacyclic monoterpenoid indole alkaloid glycoside named secorubenine (1) was isolated from the heartwood of Adina rubescens, collected in Indonesia. The structure was elucidated by spectroscopic analysis and chemical modification of isolated secorubenine (1). The bioinspired enantioselective total synthesis of 1 was accomplished in 12 steps, whereafter its structure was determined and the absolute stereochemistry was confirmed.
- 著者
- Jukiya Sakamoto Mariko Kitajima Hayato Ishikawa
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.70, no.9, pp.662-668, 2022-09-01 (Released:2022-09-01)
- 参考文献数
- 34
- 被引用文献数
- 8
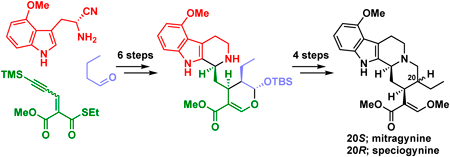
A number of alkaloids found in Mitragyna species belonging to the Rubiaceae family have been shown to have potent biological activity such as analgesic properties. Here, we report the asymmetric total syntheses of mitragynine, speciogynine, and 7-hydroxymitragynine, which are classified as corynantheine-type monoterpenoid indole alkaloids, isolated from Mitragyna speciosa. These syntheses were accomplished within 12 steps and in >11% total yield from commercial 3-(trimethylsilyl)propanal using an organocatalytic anti-selective Michael reaction and bioinspired transformations.