- 著者
- Edjohn Aaron Macauyag Hiroyuki Kajiura Takao Ohashi Ryo Misaki Kazuhito Fujiyama
- 出版者
- Japanese Society for Plant Biotechnology
- 雑誌
- Plant Biotechnology (ISSN:13424580)
- 巻号頁・発行日
- vol.39, no.3, pp.291-301, 2022-09-25 (Released:2022-09-25)
- 参考文献数
- 56
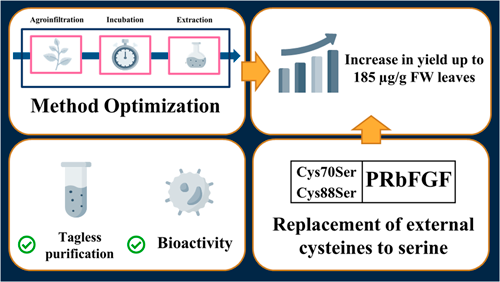
The human basic fibroblast growth factor (bFGF) is a protein that plays a pivotal role in cellular processes like cell proliferation and development. As a result, it has become an important component in cell culture systems, with applications in biomedical engineering, cosmetics, and research. Alternative production techniques, such as transient production in plants, are becoming a feasible option as the demand continues to grow. High-level bFGF production was achieved in this study employing an optimized Agrobacterium-mediated transient expression system, which yielded about a 3-fold increase in production over a conventional system. This yield was further doubled at about 185 µg g−1 FW using a mutant protease-resistant version that degraded/aggregated at a three-fold slower rate in leaf crude extracts. To achieve a pure product, a two-step purification technique was applied. The capacity of the pure protease-resistant bFGF (PRbFGF) to stimulate cell proliferation was tested and was found to be comparable to that of E. coli-produced bFGF in HepG2 and CHO-K1 cells. Overall, this study demonstrates a high-level transient production system of functional PRbFGF in N. benthamiana leaves as well as an efficient tag-less purification technique of leaf crude extracts.
- 著者
- Ratna Sariyatun Hiroyuki Kajiura Juthamard Limkul Ryo Misaki Kazuhito Fujiyama
- 出版者
- Japanese Society for Plant Biotechnology
- 雑誌
- Plant Biotechnology (ISSN:13424580)
- 巻号頁・発行日
- vol.38, no.4, pp.463-467, 2021-12-25 (Released:2021-12-25)
- 参考文献数
- 25
- 被引用文献数
- 1
N-Glycosylation is essential for protein stability, activity and characteristics, and is often needed to deliver pharmaceutical glycoproteins to target cells. A paucimannosidic structure, Man3GlcNAc2 (M3), has been reported to enable cellular uptake of glycoproteins through the mannose receptor (MR) in humans, and such uptake has been exploited for the treatment of certain diseases. However, M3 is generally produced at a very low level in plants. In this study, a cell culture was established from an Arabidopsis alg3 mutant plant lacking asparagine-linked glycosylation 3 (ALG3) enzyme activity. Arabidopsis alg3 cell culture produced glycoproteins with predominantly M3 and GlcNAc-terminal structures, while the amount of plant-specific N-glycans was very low. Pharmaceutical glycoproteins with these characteristics would be valuable for cellular delivery through the MR, and safe for human therapy.
- 著者
- Hiroyuki Kajiura Kyoko Hiwasa-Tanase Hiroshi Ezura Kazuhito Fujiyama
- 出版者
- Japanese Society for Plant Cell and Molecular Biology
- 雑誌
- Plant Biotechnology (ISSN:13424580)
- 巻号頁・発行日
- vol.35, no.4, pp.375-379, 2018-12-25 (Released:2018-12-31)
- 参考文献数
- 17
- 被引用文献数
- 1 4
Miraculin is a promising protein with taste-modifying properties. Focusing on the unique function and potential of miraculin, recombinant miraculin production has been explored with the use of heterologous expression systems, but the activities of recombinant miraculins were much lower than those of native miraculin, probably due to the difference in post-translational modification, especially N-glycosylation. For practical use therefore, the differences between N-glycan of recombinant miraculin compared to that of native miraculin should be minimized. Here, to establish the platform for functional miraculin production, we expressed miraculin in tomato plants with the same taste-modifying activity as native miraculin purified from miracle fruit, and we compared the N-glycan structures with those of native miraculin. Our N-glycan structural analysis using purified miraculin, followed by hydrazynolysis, 2-pyridylamine (PA)-labeling, high-performance liquid chromatography, and a liquid chromatography tandem-mass spectrometry analysis revealed that both the native and recombinant miraculins carried an M3 structure as a predominant structure and that most of the N-glycan structures on the miraculins were pauci-mannosidic structures with a smaller amount of plant-specific α1,3-fucosylated and/or β1,2-xylosylated N-glycans and without a Lewis a epitope. These results indicate that the N-glycoform of native miraculin from miracle fruit and recombinant miraculin expressed in tomato plants are almost identical to each other with similar ratios and that, therefore, plant-specific N-glycans are essential for showing the full taste-modifying activity of miraculin.