- 著者
- Kei Kawada Tomoaki Ishida Kohei Jobu Tsuyoshi Ohta Hitoshi Fukuda Shumpei Morisawa Tetsushi Kawazoe Naohisa Tamura Mitsuhiko Miyamura
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.45, no.6, pp.720-723, 2022-06-01 (Released:2022-06-01)
- 参考文献数
- 24
- 被引用文献数
- 3
Aggression is the most common adverse effect of antiepileptic drugs (AEDs). This study aimed to investigate the association of aggression with AED use. The reporting odds ratio (ROR) from adverse event reports, submitted to the Japanese Adverse Drug Event Report database between 2004 and 2020, was used to calculate and investigate the association between AEDs and aggression. We also analyzed the association of aggression with the combined use of AEDs and the relationship between AED-associated aggression and patient characteristics. A total of 433 patients developed aggression. Significant aggression signals were detected for perampanel (crude ROR: 325.04, 95% confidence interval (CI): 118.48–752.58, p < 0.01), levetiracetam (crude ROR: 17.14, 95% CI: 10.33–26.90, p < 0.01), lacosamide (crude ROR: 16.90, 95% CI: 2.02–62.51, p < 0.01), lamotrigine (crude ROR: 15.98, 95% CI: 9.99–24.39, p < 0.01), valproate (crude ROR: 6.68, 95% CI: 4.27–10.02, p < 0.01), and carbamazepine (crude ROR: 2.47, 95% CI: 1.17–4.59, p < 0.01). The combined therapy with perampanel and levetiracetam had a significant aggression signal (adjusted ROR: 25.90, 95% CI: 1.14–59.10, p < 0.01). In addition, we found that aggression frequently occurred in patients <60 year (adjusted ROR: 2.88, 95% CI: 1.49–5.56, p < 0.01) treated with levetiracetam. These results may be useful for minimizing the risk of aggression during the treatment of AEDs.
- 著者
- Tomoaki Ishida Kei Kawada Shumpei Morisawa Kohei Jobu Yasuyo Morita Mitsuhiko Miyamura
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.43, no.10, pp.1570-1576, 2020-10-01 (Released:2020-10-01)
- 参考文献数
- 24
- 被引用文献数
- 1 18
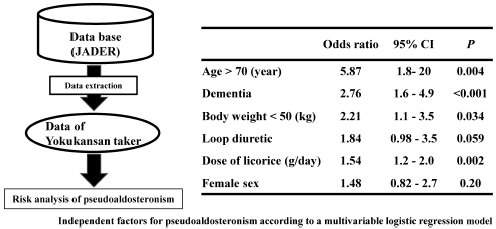
Yokukansan is a Kampo formula that is commonly used by the elderly because it is expected to improve peripheral symptoms of dementia and delirium. However, side effects from its use are frequently reported in the elderly. In particular, pseudoaldosteronism caused by the licorice contained in yokukansan leads to hypertension, hypokalemia, and muscle weakness, which may result in death. This study aimed to identify the risk factors of pseudoaldosteronism with yokukansan use. Using cases reported in the Japanese Adverse Drug Report (JADER) database, the reporting odds ratio (ROR) was calculated and compared to assess the risk of pseudoaldosteronism for each licorice-containing Kampo formula. We also analyzed the risk factors for pseudoaldosteronism in patients taking yokukansan. Yokukansan (ROR 2.4, 95% confidence interval (CI) 1.9–2.8; p < 0.001) had a higher risk of pseudoaldosteronism than that of other licorice-containing Kampo formulas. Furthermore, the results of a logistic regression analysis in patients taking yokukansan showed that the licorice dose (OR 1.5, 95% CI 1.2–2.0; p < 0.01), older age (<70 years, OR 5.9, 95% CI 1.8–20; p < 0.01), dementia (OR 2.8, 95% CI 1.6–4.9; p < 0.001), low body weight (<50 kg, OR 2.2, 95% CI 1.1–3.5; p = 0.034) were risk factors for pseudoaldosteronism, Although not significant, treatment with loop diuretics (OR 1.8, 95% CI 0.98–3.5; p = 0.059) tended to increase the risk of pseudoaldosteronism. In summary, patients must understand the risk factors when considering taking yokukansan and reduce the licorice dose they consume.
- 著者
- Tomoaki Ishida Kei Kawada Kohei Jobu Tetsushi Kawazoe Naohisa Tamura Mitsuhiko Miyamura
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.45, no.4, pp.460-466, 2022-04-01 (Released:2022-04-01)
- 参考文献数
- 34
- 被引用文献数
- 2
Bofutsushosan is a traditional Japanese Kampo medicine. In recent years, it has been reported to be effective in the treatment of lifestyle-related diseases, and its use is increasing. However, side effects from bofutsushosan administration are common, with drug-induced liver injury being the most frequently reported complication. In this study, we analyzed the Japanese Adverse Drug Event Report (JADER) database regarding the occurrence of liver injury after bofutsushosan administration. The results showed that bofutsushosan presented a significant reporting odds ratio (ROR) signal [crude ROR 14, 95% confidence interval (CI) 12–17; p < 0.001], indicating liver injury. Furthermore, the incidents of adverse events following bofutsushosan administration, as recorded in the JADER database, were higher in women aged between 30 and 59 years. The results of logistic regression analysis in patients taking this agent showed that females in the aforementioned age range had higher odds of developing drug-induced liver injury (adjusted ROR 5.5, 95% CI 2.8–11; p < 0.001). Therefore, although bofutsushosan is a useful drug for lifestyle-related diseases, it may be necessary to refrain from its overuse, and caution should be taken during its occasional use to avoid severe adverse events.