- 著者
- Tassadit Belabbas Takaaki Yamada Yuichi Tsuchiya Kimitaka Suetsugu Nobuaki Egashira Ichiro Ieiri
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.69, no.7, pp.646-651, 2021-07-01 (Released:2021-07-01)
- 参考文献数
- 17
- 被引用文献数
- 2
With the aim of studying the pharmacokinetics of letermovir, which is a newly developed antiviral agent for human cytomegalovirus, a rapid and simple ultra-performance liquid chromatography coupled with mass spectrometry (UPLC/MS) method was developed and validated for the quantification of letermovir in human plasma. Separation was performed in reverse phase mode using an ACQUITY UPLC BEH C18 column (130 Å, 1.7 µm, 2.1 × 50 mm) at a flow rate of 0.3 mL/min, 10 mM ammonium acetate–0.1% formic acid solution as mobile phase A, and acetonitrile as mobile phase B with a gradient elution. The method was validated over a linear range of 10–1000 ng/mL with a coefficient of determination (R2) >0.99 using weighted linear regression analysis. The intra- and inter-assay accuracy (nominal%) and precision (relative standard deviation%) were within ±15 and ≤15%, respectively. The specificity, recovery, matrix effect, stability, and dilution integrity of this method were also within acceptable limits. This method could be useful in studying the pharmacokinetics and pharmacodynamics, as well as performing the therapeutic drug monitoring of letermovir.
- 著者
- Takanori Mei Hiroshi Noguchi Kimitaka Suetsugu Yu Hisadome Keizo Kaku Yasuhiro Okabe Satohiro Masuda Masafumi Nakamura
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.43, no.10, pp.1600-1603, 2020-10-01 (Released:2020-10-01)
- 参考文献数
- 16
- 被引用文献数
- 3
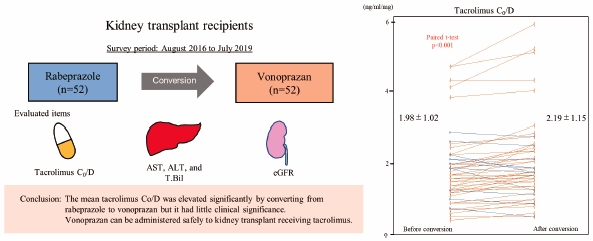
Vonoprazan fumarate (vonoprazan) is a new kind of acid suppressant with potent acid inhibitory effects. Therefore, it has been administered to kidney transplant recipients for treatment or prophylaxis of steroid ulcers, refractory peptic ulcers, and gastroesophageal reflux disease. Because tacrolimus, which is a well-established immunosuppressant for kidney transplantation, and vonoprazan share the CYP3A4 system for metabolism, drug interactions are anticipated upon simultaneous administration. We retrospectively analyzed 52 kidney transplant recipients who were converted from rabeprazole, which has a small effect on the tacrolimus trough blood concentration (C0), to vonoprazan between August 2016 and July 2019. We compared the tacrolimus C0/tacrolimus dose (C0/D) before and after conversion and serum liver enzymes, serum total bilirubin, and the estimated glomerular filtration rate (eGFR). As a result, mean tacrolimus C0/D before and after conversion was 1.98 ± 1.02 and 2.19 ± 1.15 (ng/mL)/(mg/d), respectively, (p < 0.001). Additionally, mean aspartate transaminase (AST) before and after conversion was 18.6 ± 4.2 and 19.6 ± 5.2 IU/L, respectively, (p = 0.037). Mean alanine transaminase (ALT) before and after conversion was 15.8 ± 5.5 and 17.6 ± 7.1 IU/L, respectively, (p = 0.007). Mean eGFR before and after conversion was 50.6 ± 14.4 and 51.4 ± 14.7 mL/min/1.73 m2, respectively (p = 0.021). Mean AST, ALT, and eGFR were slightly but significantly elevated within normal ranges after conversion. In conclusion, our study suggests that the mean tacrolimus C0/D was elevated significantly by converting from rabeprazole to vonoprazan, but it had little clinical significance. Vonoprazan can be administered safely to kidney transplant recipients receiving tacrolimus.