- 著者
- Yuya Matsuda Shunsaku Nakagawa Ikuko Yano Satohiro Masuda Satoshi Imai Atsushi Yonezawa Takashi Yamamoto Mitsuhiro Sugimoto Masahiro Tsuda Tetsunori Tsuzuki Tomohiro Omura Takayuki Nakagawa Toyofumi Fengshi Chen-Yoshikawa Miki Nagao Hiroshi Date Kazuo Matsubara
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.45, no.4, pp.397-402, 2022-04-01 (Released:2022-04-01)
- 参考文献数
- 24
- 被引用文献数
- 3
Invasive Aspergillus infection is a major factor for poor prognosis in patients receiving lung transplantation (LT). An antifungal agent, itraconazole (ITCZ), that has antimicrobial activity against Aspergillus species, is used as a prophylactic agent against Aspergillus infection after LT. ITCZ and its metabolite, hydroxyitraconazole (OH-ITCZ), potently inhibit CYP3A and P-glycoprotein that metabolize or excrete calcineurin inhibitors (CNIs), which are the first-line immunosuppressants used after LT; thus, concomitant use of ITCZ and CNIs could induce an increase in the blood concentration of CNIs. However, no criteria for dose reduction of CNIs upon concomitant use with ITCZ in LT recipients have been defined. In this study, the effect of ITCZ and OH-ITCZ on the blood concentrations of two CNIs, tacrolimus and cyclosporine, after LT were retrospectively evaluated. A total of 39 patients who received LT were evaluated. Effects of ITCZ and OH-ITCZ on the concentration/dosage (C/D) ratio of tacrolimus and cyclosporine were analyzed using linear mixed-effects models. The plasma concentrations of OH-ITCZ were about 2.5-fold higher than those of ITCZ. Moreover, there was a significant correlation between the plasma concentrations of ITCZ and OH-ITCZ. Based on parameters obtained in the linear regression analysis, the C/D ratios of cyclosporine and tacrolimus increase by an average of 2.25- and 2.70-fold, respectively, when the total plasma concentration of ITCZ plus OH-ITCZ is 1000 ng/mL. In conclusion, the plasma levels of ITCZ and OH-ITCZ could be key factors in drawing up the criterion for dose reduction of CNIs.
- 著者
- Shigeru Ishida Hanae Morikawa Hiroyuki Watanabe Toshikazu Tsuji Takeshi Sugio Yasuo Mori Toshihiro Miyamoto Satohiro Masuda Koichi Akashi Nobuaki Egashira
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.43, no.3, pp.488-492, 2020-03-01 (Released:2020-03-01)
- 参考文献数
- 19
- 被引用文献数
- 1
The intravenous injection of bendamustine often induces venous irritation, which reduces patients’ QOL. We previously reported that the dilution of the final volume of bendamustine from 250 to 500 mL significantly decreased the incidence of venous irritation. However, the influence of this change on the therapeutic efficacy of bendamustine remains unclear. Therefore, the aim of this study was to evaluate the efficacy and safety profiles of bendamustine at different dilutions of the final volume, comparing with the correspondences of previous studies. Thirty-four patients, who received a total of 161 courses of bendamustine and rituximab chemotherapy, were included in this study. The overall response rate of this regimen was 94.1% in this study, which was comparable to that reported in the BRB study (94.2%, a phase II study of bendamustine plus rituximab therapy in Japanese patients). Additionally, the median progression-free survival was not inferior to that reported in the BRB study. Bendamustine-induced venous irritation was observed in 17.6% of the patients during the first treatment cycle administered at a final volume of 500 mL, and was found to be lower than that observed in the control, where bendamustine was administered at a final volume of 250 mL (85.7%). These results suggest that diluting bendamustine to 500 mL, but not to 250 mL, reduces the incidence of venous irritation without a negative impact on its therapeutic efficacy; thus, this simple strategy may be beneficial to ensure efficacy and safety in patients receiving regimens including bendamustine.
- 著者
- Eriko SATO Ikuko YANO Masahiro SHIMOMURA Satohiro MASUDA Toshiya KATSURA Shin-ichi MATSUMOTO Teru OKITSU Yasuhiro IWANAGA Shinji UEMOTO Ken-ichi INUI
- 出版者
- The Japanese Society for the Study of Xenobiotics
- 雑誌
- Drug Metabolism and Pharmacokinetics (ISSN:13474367)
- 巻号頁・発行日
- vol.24, no.2, pp.175-179, 2009 (Released:2009-05-10)
- 参考文献数
- 20
- 被引用文献数
- 3
We attempted a switch of mammalian target of rapamycin (mTOR) inhibitors from sirolimus to everolimus, a derivative of sirolimus and now on the market in Japan, in two pancreatic islet transplant patients. Both patients were administered tacrolimus with sirolimus or everolimus. They had been administered 5 or 9 mg sirolimus once a day and had maintained a trough concentration of about 15 ng/mL as measured by high performance liquid chromatography with ultraviolet detection. After the switch from sirolimus to everolimus, they were given 10 or 12 mg/day of everolimus twice a day to maintain a trough concentration of 12-15 ng/mL as measured by a fluorescence polarization immunoassay (FPIA) method. Afterward, the blood concentrations of everolimus and sirolimus after the conversion were measured by high performance liquid chromatography with mass spectrometry and everolimus concentrations were found to be 5-10 ng/mL. These data show that a larger dosage is needed for everolimus than sirolimus to maintain the same trough blood concentration. Data obtained by the FPIA for everolimus should be carefully evaluated after switching from sirolimus to everolimus because of the cross-reactivity of the antibody with sirolimus.
- 著者
- Shigeru Ishida Ken Masuguchi Takehiro Kawashiri Toshikazu Tsuji Hiroyuki Watanabe Sayuri Akiyoshi Makoto Kubo Satohiro Masuda Nobuaki Egashira
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.43, no.4, pp.663-668, 2020-04-01 (Released:2020-04-01)
- 参考文献数
- 38
- 被引用文献数
- 1 2
Hypersensitivity reactions, including anaphylaxis, are common side effects associated with docetaxel treatment in breast cancer patients. However, preventive measures have not yet been established. In this study, we retrospectively analyzed the risk factors for developing anaphylaxis in 182 female breast cancer patients treated with docetaxel. We found that 6.6% of all patients (n = 12) experienced anaphylaxis. Multivariate analyses indicated that concentration of docetaxel higher than 0.275 mg/m2/mL, docetaxel dose rate higher than 1.15 mg/m2/min, and white blood cell count less than 4290 cells/mL are risk factors for developing docetaxel-related anaphylaxis. In particular, concentrations of docetaxel or doses per administration time were associated with a high odds ratio (11.88 or 11.60) for docetaxel-related anaphylaxis. Moreover, patients receiving doses in 250 mL volume experienced anaphylaxis more frequently than those receiving doses in 500 mL (7.0 vs. 0.9%, p = 0.0236). Additionally, patients receiving treatments over 60 min tended to experience anaphylaxis more frequently than those who were treated over 90 min (6.7 vs. 1.1%, p = 0.0637). The present results indicate that high docetaxel concentrations, high dose rates, and low white blood cell counts are risk factors for developing docetaxel-related anaphylaxis, and administering docetaxel diluted in 500 mL over 90 min may limit docetaxel-induced hypersensitivity reactions.
- 著者
- Takanori Mei Hiroshi Noguchi Kimitaka Suetsugu Yu Hisadome Keizo Kaku Yasuhiro Okabe Satohiro Masuda Masafumi Nakamura
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.43, no.10, pp.1600-1603, 2020-10-01 (Released:2020-10-01)
- 参考文献数
- 16
- 被引用文献数
- 3
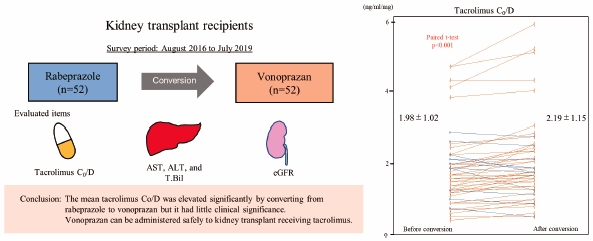
Vonoprazan fumarate (vonoprazan) is a new kind of acid suppressant with potent acid inhibitory effects. Therefore, it has been administered to kidney transplant recipients for treatment or prophylaxis of steroid ulcers, refractory peptic ulcers, and gastroesophageal reflux disease. Because tacrolimus, which is a well-established immunosuppressant for kidney transplantation, and vonoprazan share the CYP3A4 system for metabolism, drug interactions are anticipated upon simultaneous administration. We retrospectively analyzed 52 kidney transplant recipients who were converted from rabeprazole, which has a small effect on the tacrolimus trough blood concentration (C0), to vonoprazan between August 2016 and July 2019. We compared the tacrolimus C0/tacrolimus dose (C0/D) before and after conversion and serum liver enzymes, serum total bilirubin, and the estimated glomerular filtration rate (eGFR). As a result, mean tacrolimus C0/D before and after conversion was 1.98 ± 1.02 and 2.19 ± 1.15 (ng/mL)/(mg/d), respectively, (p < 0.001). Additionally, mean aspartate transaminase (AST) before and after conversion was 18.6 ± 4.2 and 19.6 ± 5.2 IU/L, respectively, (p = 0.037). Mean alanine transaminase (ALT) before and after conversion was 15.8 ± 5.5 and 17.6 ± 7.1 IU/L, respectively, (p = 0.007). Mean eGFR before and after conversion was 50.6 ± 14.4 and 51.4 ± 14.7 mL/min/1.73 m2, respectively (p = 0.021). Mean AST, ALT, and eGFR were slightly but significantly elevated within normal ranges after conversion. In conclusion, our study suggests that the mean tacrolimus C0/D was elevated significantly by converting from rabeprazole to vonoprazan, but it had little clinical significance. Vonoprazan can be administered safely to kidney transplant recipients receiving tacrolimus.