- 著者
- Natsumi Seki Masahiro Akiyama Hiroto Yamakawa Koji Hase Yoshito Kumagai Yun-Gi Kim
- 出版者
- The Japanese Society of Toxicology
- 雑誌
- The Journal of Toxicological Sciences (ISSN:03881350)
- 巻号頁・発行日
- vol.46, no.2, pp.91-97, 2021 (Released:2021-02-02)
- 参考文献数
- 29
- 被引用文献数
- 5 12
Methylmercury (MeHg), an environmental electrophile, binds covalently to the cysteine residues of proteins in organs, altering protein function and causing cytotoxicity. MeHg has also been shown to alter the composition of gut microbes. The gut microbiota is a complex community, the disturbance of which has been linked to the development of certain diseases. However, the relationship between MeHg and gut bacteria remains poorly understood. In this study, we showed that MeHg binds covalently to gut bacterial proteins via cysteine residues. We examined the effects of MeHg on the growth of selected Lactobacillus species, namely, L. reuteri, L. gasseri, L. casei, and L. acidophilus, that are frequently either positively or negatively correlated with human diseases. The results revealed that MeHg inhibits the growth of Lactobacillus to varying degrees depending on the species. Furthermore, the growth of L. reuteri, which was inhibited by MeHg exposure, was restored by Na2S2 treatment. By comparing mice with and without gut microbiota colonization, we found that gut bacteria contribute to the production of reactive sulfur species such as hydrogen sulfide and hydrogen persulfide in the gut. We also discovered that the removal of gut bacteria accelerated accumulation of mercury in the cerebellum, liver, and lungs of mice subsequent to MeHg exposure. These results accordingly indicate that MeHg is captured and inactivated by the hydrogen sulfide and hydrogen persulfide produced by intestinal microbes, thereby providing evidence for the role played by gut microbiota in reducing MeHg toxicity.
- 著者
- Wei Zhe Naomi Hoshina Yukihiro Itoh Toshifumi Tojo Takayoshi Suzuki Koji Hase Daisuke Takahashi
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.45, no.9, pp.1364-1372, 2022-09-01 (Released:2022-09-01)
- 参考文献数
- 22
- 被引用文献数
- 1
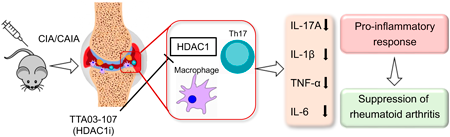
Rheumatoid arthritis (RA) is systemic autoimmune arthritis that causes joint inflammation and destruction. Accumulating evidence has shown that inhibitors of class I histone deacetylases (HDACs) (i.e., HDAC1, 2, 3, and 8) are potential therapeutic candidates as targeted synthetic disease-modifying antirheumatic drugs (tsDMARDs). Nevertheless, the inhibition of class I HDACs has severe adverse effects because of their broad spectrum. We evaluated the therapeutic effect of a novel selective HDAC1 inhibitor TTA03-107 for collagen-induced arthritis (CIA) and collagen antibody-induced arthritis (CAIA) models in mice. We also examined the effect of TTA03-107 in bone marrow-derived macrophages (BMDMs) and T helper 17 (Th17) cells in vitro. Here, we delineate that TTA03-107 reduced the severity of autoimmune arthritis without obvious adverse effects in CIA and CAIA models. Moreover, TTA03-107 suppressed the production of inflammatory cytokines, such as interleukin (IL)-1β, tumor necrosis factor (TNF)-α, and IL-17A, in serum and joint tissue. In vitro treatment of BMDMs with TTA03-107 dampened the M1 differentiation and inflammatory cytokine production. TTA03-107 also suppressed the differentiation of Th17 cells. These results demonstrate that TTA03-107 can attenuate the development of arthritis in experimental RA models by inhibiting the differentiation and activation of macrophages and Th17 cells. Therefore, TTA03-107 is a potential tsDMARD candidate.
1 0 0 0 OA Intestinal epithelial cell-specific deletion of α-mannosidase II ameliorates experimental colitis
- 著者
- Koichiro Suzuki Takahiro Yamada Keiko Yamazaki Masato Hirota Narumi Ishihara Mizuki Sakamoto Daisuke Takahashi Hideki Iijima Koji Hase
- 出版者
- Japan Society for Cell Biology
- 雑誌
- Cell Structure and Function (ISSN:03867196)
- 巻号頁・発行日
- pp.17022, (Released:2018-01-18)
- 被引用文献数
- 9
Inflammatory bowel disease (IBD) is a refractory disease of the gastrointestinal tract that is believed to develop in genetically susceptible individuals. Glycosylation, a type of post-translational modification, is involved in the development of a wide range of diseases, including IBD, by modulating the function of various glycoproteins. To identify novel genes contributing to the development of IBD, we analyzed single nucleotide polymorphisms (SNPs) of glycosylation-related genes in IBD patients and identified MAN2A1, encoding alpha-mannosidase II (α-MII), as a candidate gene. α-MII plays a crucial, but not exclusive, role in the maturation of N-glycans. We also observed that intestinal epithelial cells (IECs), which establish the first-line barrier and regulate gut immunity, selectively expressed α-MII with minimal expression of its isozyme, alpha-mannosidase IIx (α-MIIx). This led us to hypothesize that IEC-intrinsic α-MII is implicated in the pathogenesis of IBD. To test this hypothesis, we generated IEC-specific α-MII-deficient (α-MIIΔIEC) mice. Although α-MII deficiency has been shown to have a minimal effect on N-glycan maturation in most cell types due to the compensation by α-MIIx, ablation of α-MII impaired the maturation of N-glycans in IECs. α-MIIΔIEC mice were less susceptible to dextran sulfate sodium-induced colitis compared with control littermates. In accordance with this, neutrophil infiltration in the colonic mucosa was attenuated in α-MIIΔIEC mice. Furthermore, gene expression levels of neutrophil-attracting chemokines were downregulated in the colonic tissue. These results suggest that IEC-intrinsic α-MII promotes intestinal inflammation by facilitating chemokine expression. We propose SNPs in MAN2A1 as a novel genetic factor for IBD. Key words: inflammatory bowel disease, alpha-mannosidase II, intestinal epithelial cell, N-glycosylation