- 著者
- Daniel Dai Muneyoshi Ichikawa Katya Peri Reid Rebinsky Khanh Huy Bui
- 出版者
- The Biophysical Society of Japan
- 雑誌
- Biophysics and Physicobiology (ISSN:21894779)
- 巻号頁・発行日
- vol.17, pp.71-85, 2020 (Released:2020-07-22)
- 参考文献数
- 48
- 被引用文献数
- 13 28
Cilia or flagella of eukaryotes are small micro-hair like structures that are indispensable to single-cell motility and play an important role in mammalian biological processes. Cilia or flagella are composed of nine doublet microtubules surrounding a pair of singlet microtubules called the central pair (CP). Together, this arrangement forms a canonical and highly conserved 9+2 axonemal structure. The CP, which is a unique structure exclusive to motile cilia, is a pair of structurally dimorphic singlet microtubules decorated with numerous associated proteins. Mutations of CP-associated proteins cause several different physical symptoms termed as ciliopathies. Thus, it is crucial to understand the architecture of the CP. However, the protein composition of the CP was poorly understood. This was because the traditional method of identification of CP proteins was mostly limited by available Chlamydomonas mutants of CP proteins. Recently, more CP protein candidates were presented based on mass spectrometry results, but most of these proteins were not validated. In this study, we re-evaluated the CP proteins by conducting a similar comprehensive CP proteome analysis comparing the mass spectrometry results of the axoneme sample prepared from Chlamydomonas strains with and without CP complex. We identified a similar set of CP protein candidates and additional new 11 CP protein candidates. Furthermore, by using Chlamydomonas strains lacking specific CP sub-structures, we present a more complete model of localization for these CP proteins. This work has established a new foundation for understanding the function of the CP complex in future studies.
- 著者
- Toshiki Yagi Akiyuki Toda Muneyoshi Ichikawa Genji Kurisu
- 出版者
- The Biophysical Society of Japan
- 雑誌
- Biophysics and Physicobiology (ISSN:21894779)
- 巻号頁・発行日
- vol.20, no.1, pp.e200008, 2023 (Released:2023-03-04)
- 参考文献数
- 43
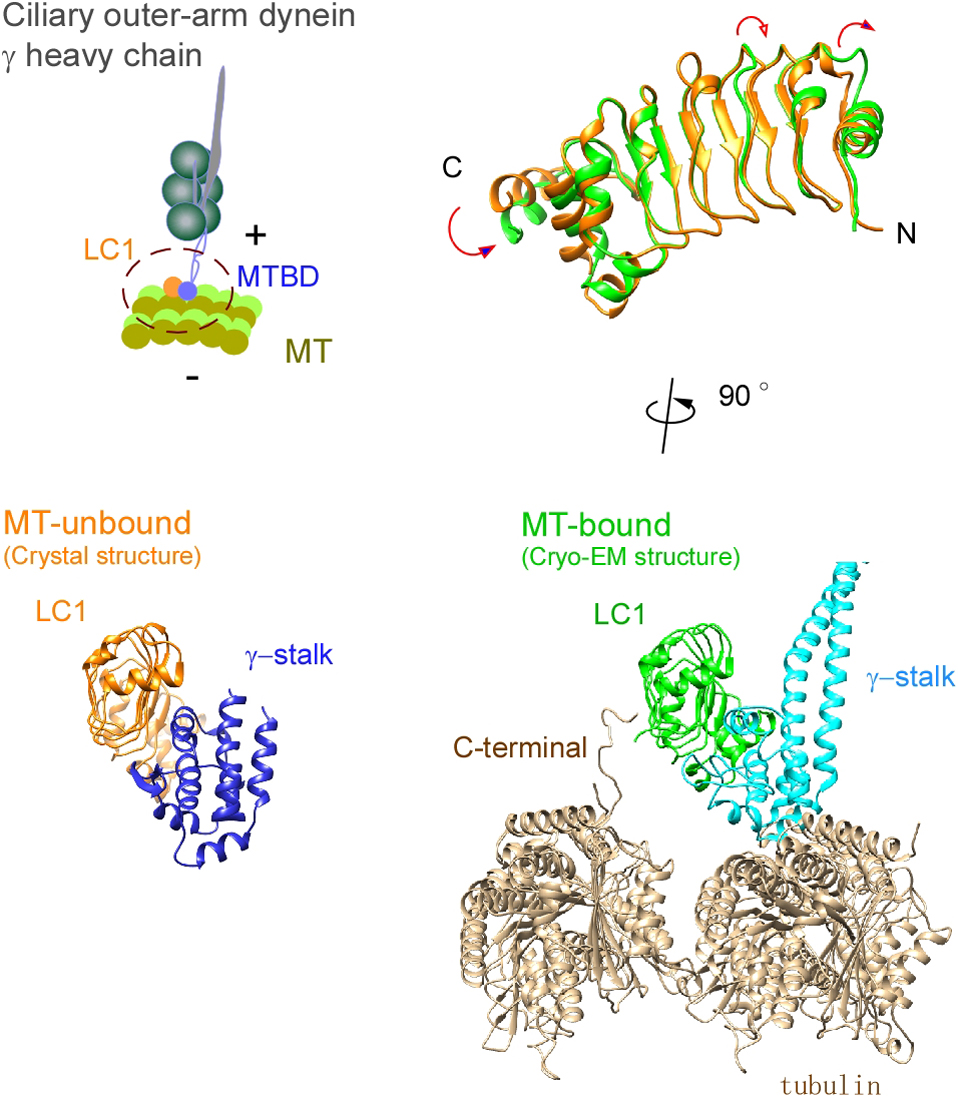
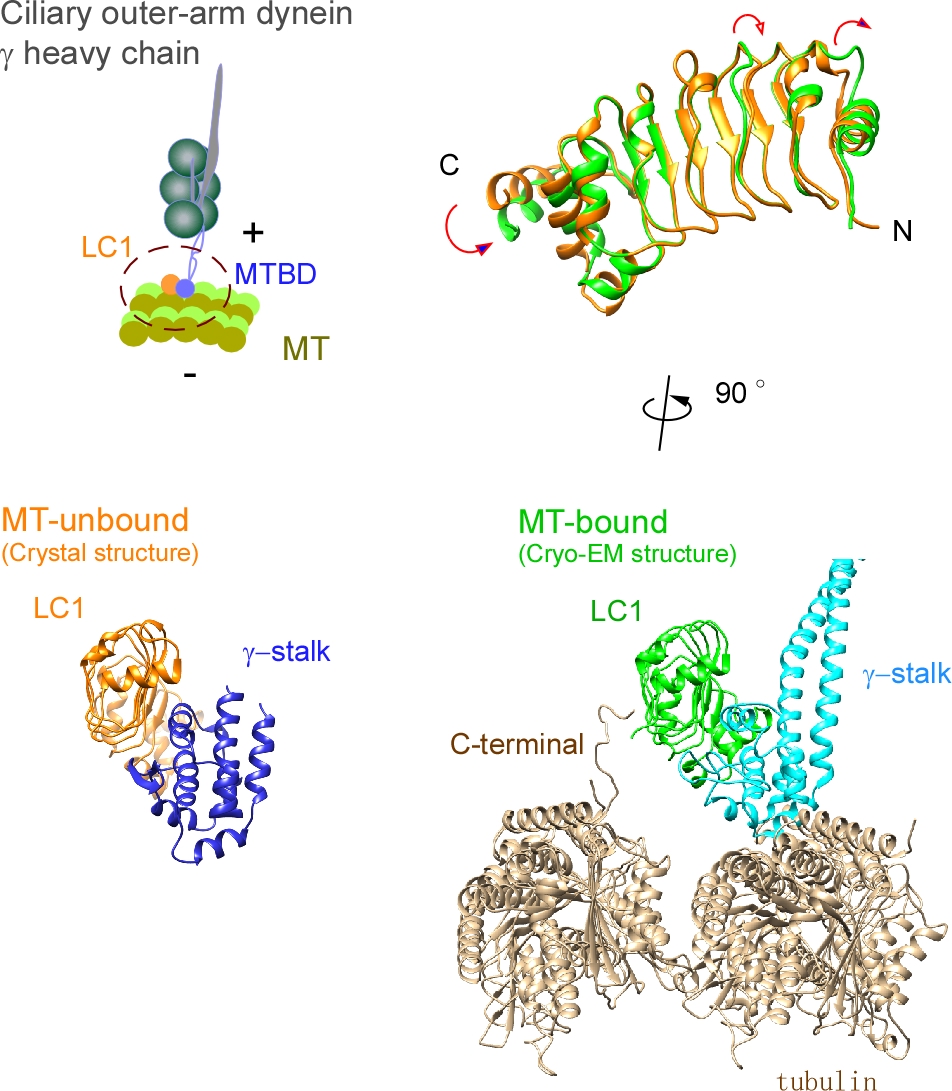
Ciliary bending movements are powered by motor protein axonemal dyneins. They are largely classified into two groups, inner-arm dynein and outer-arm dynein. Outer-arm dynein, which is important for the elevation of ciliary beat frequency, has three heavy chains (α, β, and γ), two intermediate chains, and more than 10 light chains in green algae, Chlamydomonas. Most of intermediate chains and light chains bind to the tail regions of heavy chains. In contrast, the light chain LC1 was found to bind to the ATP-dependent microtubule-binding domain of outer-arm dynein γ-heavy chain. Interestingly, LC1 was also found to interact with microtubules directly, but it reduces the affinity of the microtubule-binding domain of γ-heavy chain for microtubules, suggesting the possibility that LC1 may control ciliary movement by regulating the affinity of outer-arm dyneins for microtubules. This hypothesis is supported by the LC1 mutant studies in Chlamydomonas and Planaria showing that ciliary movements in LC1 mutants were disordered with low coordination of beating and low beat frequency. To understand the molecular mechanism of the regulation of outer-arm dynein motor activity by LC1, X-ray crystallography and cryo-electron microscopy have been used to determine the structure of the light chain bound to the microtubule-binding domain of γ-heavy chain. In this review article, we show the recent progress of structural studies of LC1, and suggest the regulatory role of LC1 in the motor activity of outer-arm dyneins. This review article is an extended version of the Japanese article, The Complex of Outer-arm Dynein Light Chain-1 and the Microtubule-binding Domain of the Heavy Chain Shows How Axonemal Dynein Tunes Ciliary Beating, published in SEIBUTSU BUTSURI Vol. 61, p. 20–22 (2021).
- 著者
- Daniel Dai Muneyoshi Ichikawa Katya Peri Reid Rebinsky Khanh Huy Bui
- 出版者
- The Biophysical Society of Japan
- 雑誌
- Biophysics and Physicobiology (ISSN:21894779)
- 巻号頁・発行日
- pp.BSJ-2019048, (Released:2020-06-16)
- 被引用文献数
- 28
- 著者
- Toshiki Yagi Akiyuki Toda Muneyoshi Ichikawa Genji Kurisu
- 出版者
- The Biophysical Society of Japan
- 雑誌
- Biophysics and Physicobiology (ISSN:21894779)
- 巻号頁・発行日
- pp.e200008, (Released:2023-02-08)
Ciliary bending movements are powered by motor protein axonemal dyneins. They are largely classified into two groups, inner-arm dynein and outer-arm dynein. Outer-arm dynein, which is important for the elevation of ciliary beat frequency, has three heavy chains (α, β, and γ), two intermediate chains, and more than 10 light chains in green algae, Chlamydomonas. Most of intermediate chains and light chains bind to the tail regions of heavy chains. In contrast, the light chain LC1 was found to bind to the ATP-dependent microtubule-binding domain of outer-arm dynein γ-heavy chain. Interestingly, LC1 was also found to interact with microtubules directly, but it reduces the affinity of the microtubule-binding domain of γ-heavy chain for microtubules, suggesting the possibility that LC1 may control ciliary movement by regulating the affinity of outer-arm dyneins for microtubules. This hypothesis is supported by the LC1 mutant studies in Chlamydomonas and Planaria showing that ciliary movements in LC1 mutants were disordered with low coordination of beating and low beat frequency. To understand the molecular mechanism of the regulation of outer-arm dynein motor activity by LC1, X-ray crystallography and cryo-electron microscopy have been used to determine the structure of the light chain bound to the microtubule-binding domain of γ-heavy chain. In this review article, we show the recent progress of structural studies of LC1, and suggest the regulatory role of LC1 in the motor activity of outer-arm dyneins. This review article is an extended version of the Japanese article, The Complex of Outer-arm Dynein Light Chain-1 and the Microtubule-binding Domain of the Heavy Chain Shows How Axonemal Dynein Tunes Ciliary Beating, published in SEIBUTSU BUTSURI Vol. 61, p.20-22 (2021).