- 著者
- Toshiki Yagi Akiyuki Toda Muneyoshi Ichikawa Genji Kurisu
- 出版者
- The Biophysical Society of Japan
- 雑誌
- Biophysics and Physicobiology (ISSN:21894779)
- 巻号頁・発行日
- vol.20, no.1, pp.e200008, 2023 (Released:2023-03-04)
- 参考文献数
- 43
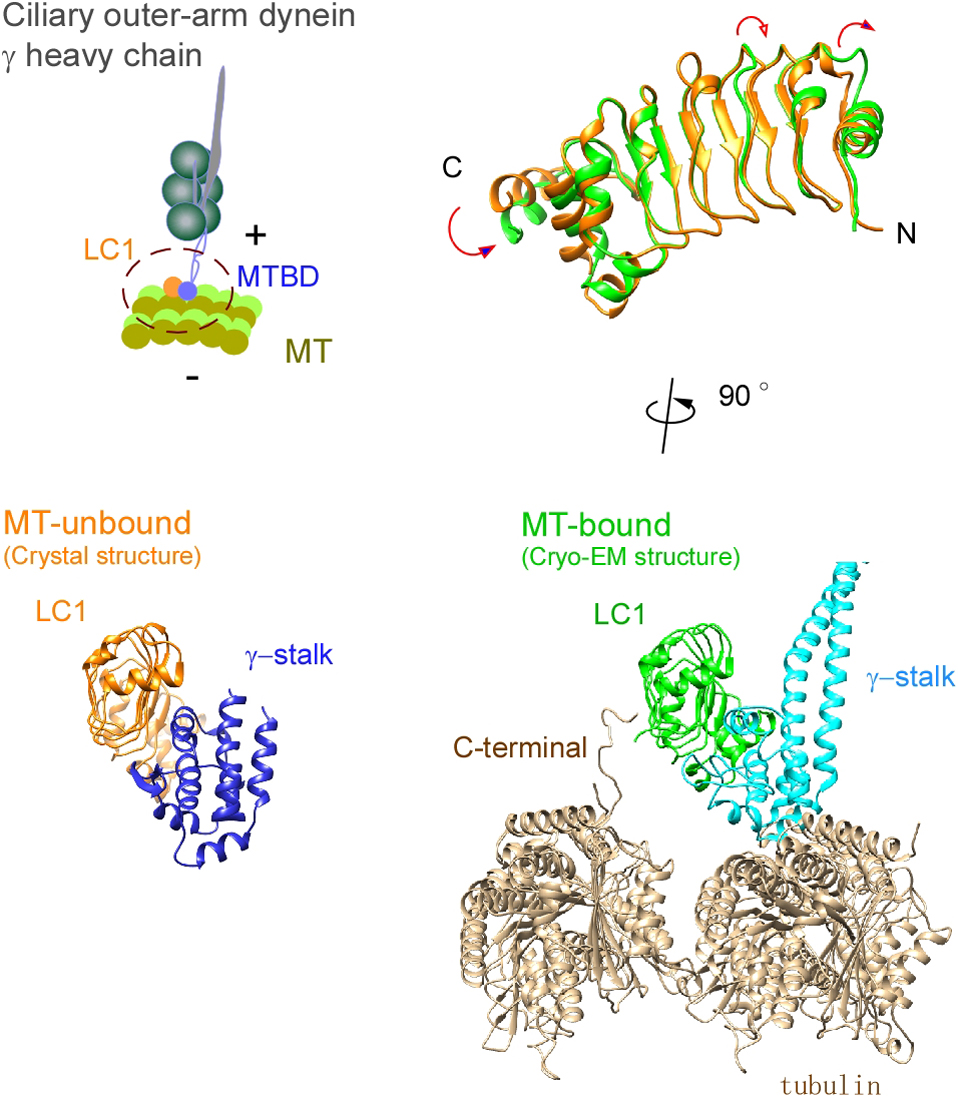
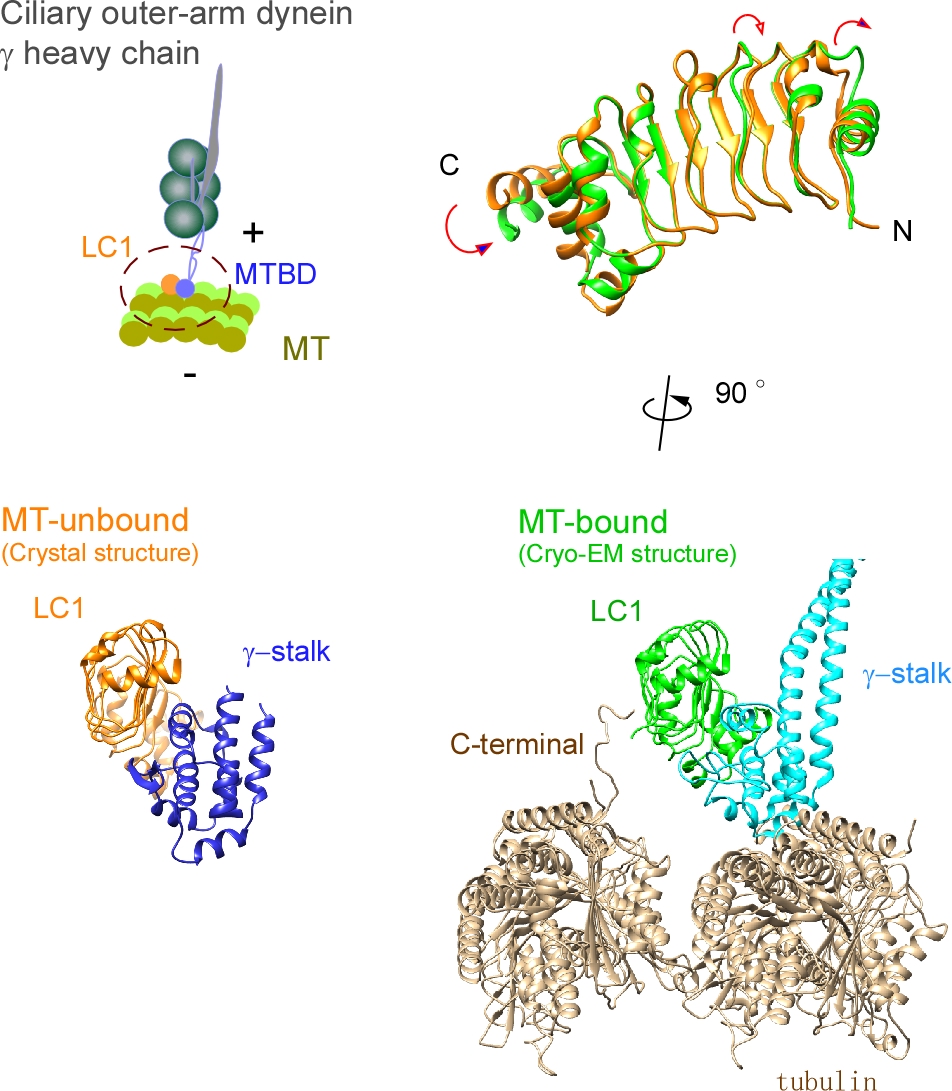
Ciliary bending movements are powered by motor protein axonemal dyneins. They are largely classified into two groups, inner-arm dynein and outer-arm dynein. Outer-arm dynein, which is important for the elevation of ciliary beat frequency, has three heavy chains (α, β, and γ), two intermediate chains, and more than 10 light chains in green algae, Chlamydomonas. Most of intermediate chains and light chains bind to the tail regions of heavy chains. In contrast, the light chain LC1 was found to bind to the ATP-dependent microtubule-binding domain of outer-arm dynein γ-heavy chain. Interestingly, LC1 was also found to interact with microtubules directly, but it reduces the affinity of the microtubule-binding domain of γ-heavy chain for microtubules, suggesting the possibility that LC1 may control ciliary movement by regulating the affinity of outer-arm dyneins for microtubules. This hypothesis is supported by the LC1 mutant studies in Chlamydomonas and Planaria showing that ciliary movements in LC1 mutants were disordered with low coordination of beating and low beat frequency. To understand the molecular mechanism of the regulation of outer-arm dynein motor activity by LC1, X-ray crystallography and cryo-electron microscopy have been used to determine the structure of the light chain bound to the microtubule-binding domain of γ-heavy chain. In this review article, we show the recent progress of structural studies of LC1, and suggest the regulatory role of LC1 in the motor activity of outer-arm dyneins. This review article is an extended version of the Japanese article, The Complex of Outer-arm Dynein Light Chain-1 and the Microtubule-binding Domain of the Heavy Chain Shows How Axonemal Dynein Tunes Ciliary Beating, published in SEIBUTSU BUTSURI Vol. 61, p. 20–22 (2021).
- 著者
- Toshiki Yagi Akiyuki Toda Muneyoshi Ichikawa Genji Kurisu
- 出版者
- The Biophysical Society of Japan
- 雑誌
- Biophysics and Physicobiology (ISSN:21894779)
- 巻号頁・発行日
- pp.e200008, (Released:2023-02-08)
Ciliary bending movements are powered by motor protein axonemal dyneins. They are largely classified into two groups, inner-arm dynein and outer-arm dynein. Outer-arm dynein, which is important for the elevation of ciliary beat frequency, has three heavy chains (α, β, and γ), two intermediate chains, and more than 10 light chains in green algae, Chlamydomonas. Most of intermediate chains and light chains bind to the tail regions of heavy chains. In contrast, the light chain LC1 was found to bind to the ATP-dependent microtubule-binding domain of outer-arm dynein γ-heavy chain. Interestingly, LC1 was also found to interact with microtubules directly, but it reduces the affinity of the microtubule-binding domain of γ-heavy chain for microtubules, suggesting the possibility that LC1 may control ciliary movement by regulating the affinity of outer-arm dyneins for microtubules. This hypothesis is supported by the LC1 mutant studies in Chlamydomonas and Planaria showing that ciliary movements in LC1 mutants were disordered with low coordination of beating and low beat frequency. To understand the molecular mechanism of the regulation of outer-arm dynein motor activity by LC1, X-ray crystallography and cryo-electron microscopy have been used to determine the structure of the light chain bound to the microtubule-binding domain of γ-heavy chain. In this review article, we show the recent progress of structural studies of LC1, and suggest the regulatory role of LC1 in the motor activity of outer-arm dyneins. This review article is an extended version of the Japanese article, The Complex of Outer-arm Dynein Light Chain-1 and the Microtubule-binding Domain of the Heavy Chain Shows How Axonemal Dynein Tunes Ciliary Beating, published in SEIBUTSU BUTSURI Vol. 61, p.20-22 (2021).
1 0 0 0 OA IC2 participates in the cooperative activation of outer arm dynein densely attached to microtubules
- 著者
- Yusuke Kondo Tomoka Ogawa Emiri Kanno Masafumi Hirono Takako Kato-Minoura Ritsu Kamiya Toshiki Yagi
- 出版者
- Japan Society for Cell Biology
- 雑誌
- Cell Structure and Function (ISSN:03867196)
- 巻号頁・発行日
- vol.48, no.2, pp.175-185, 2023 (Released:2023-09-23)
- 参考文献数
- 54
Ciliary outer-arm dynein (OAD) consists of heavy chains (HCs), intermediate chains (ICs), and light chains (LCs), of which HCs are the motor proteins that produce force. Studies using the green alga Chlamydomonas have revealed that ICs and LCs form a complex (IC/LC tower) at the base of the OAD tail and play a crucial role in anchoring OAD to specific sites on the microtubule. In this study, we isolated a novel slow-swimming Chlamydomonas mutant deficient in the IC2 protein. This mutation, E279K, is in the third of the seven WD repeat domains. No apparent abnormality was observed in electron microscope observations of axonemes or in SDS-PAGE analyses of dynein subunits. To explore the reason for the lowered motility in this mutant, in vitro microtubule sliding experiments were performed, which revealed that the motor activity of the mutant OAD was lowered. In particular, a large difference was observed between wild type (WT) and the mutant in the microtubule sliding velocity in microtubule bundles formed with the addition of OAD: ~35.3 μm/sec (WT) and ~4.3 μm/sec (mutant). From this and other results, we propose that IC2 in an OAD interacts with the β HC of the adjacent OAD, and that an OAD-OAD interaction is important for efficient beating of cilia and flagella.Key words: cilia, axoneme, dynein heavy chain, cooperativity
- 著者
- Kuniyasu SUZAKI Kengo IIJIMA Toshiki YAGI Cyrille ARTHO
- 出版者
- The Institute of Electronics, Information and Communication Engineers
- 雑誌
- IEICE TRANSACTIONS on Fundamentals of Electronics, Communications and Computer Sciences (ISSN:09168508)
- 巻号頁・発行日
- vol.E96-A, no.1, pp.215-224, 2013-01-01
- 被引用文献数
- 10
Memory deduplication improves the utilization of physical memory by sharing identical blocks of data. Although memory deduplication is most effective when many virtual machines with same operating systems run on a CPU, cross-user memory deduplication is a covert channel and causes serious memory disclosure attack. It reveals the existence of an application or file on another virtual machine. The covert channel is a difference in write access time on deduplicated memory pages that are re-created by Copy-On-Write, but it has some interferences caused by execution environments. This paper indicates that the attack includes implementation issues caused by memory alignment, self-reflection between page cache and heap, and run-time modification (swap-out, anonymous pages, ASLR, preloading mechanism, and self-modification code). However, these problems are avoidable with some techniques. In our experience on KSM (kernel samepage merging) with the KVM virtual machine, the attack could detect the security level of attacked operating systems, find vulnerable applications, and confirm the status of attacked applications.