- 著者
- Itsuki Iwamoto Ade Kurniawan Hiroki Hasegawa Yoshiaki Kashiwaya Takahiro Nomura Tomohiro Akiyama
- 出版者
- The Iron and Steel Institute of Japan
- 雑誌
- ISIJ International (ISSN:09151559)
- 巻号頁・発行日
- vol.62, no.12, pp.2483-2490, 2022-12-15 (Released:2022-12-15)
- 参考文献数
- 38
- 被引用文献数
- 2
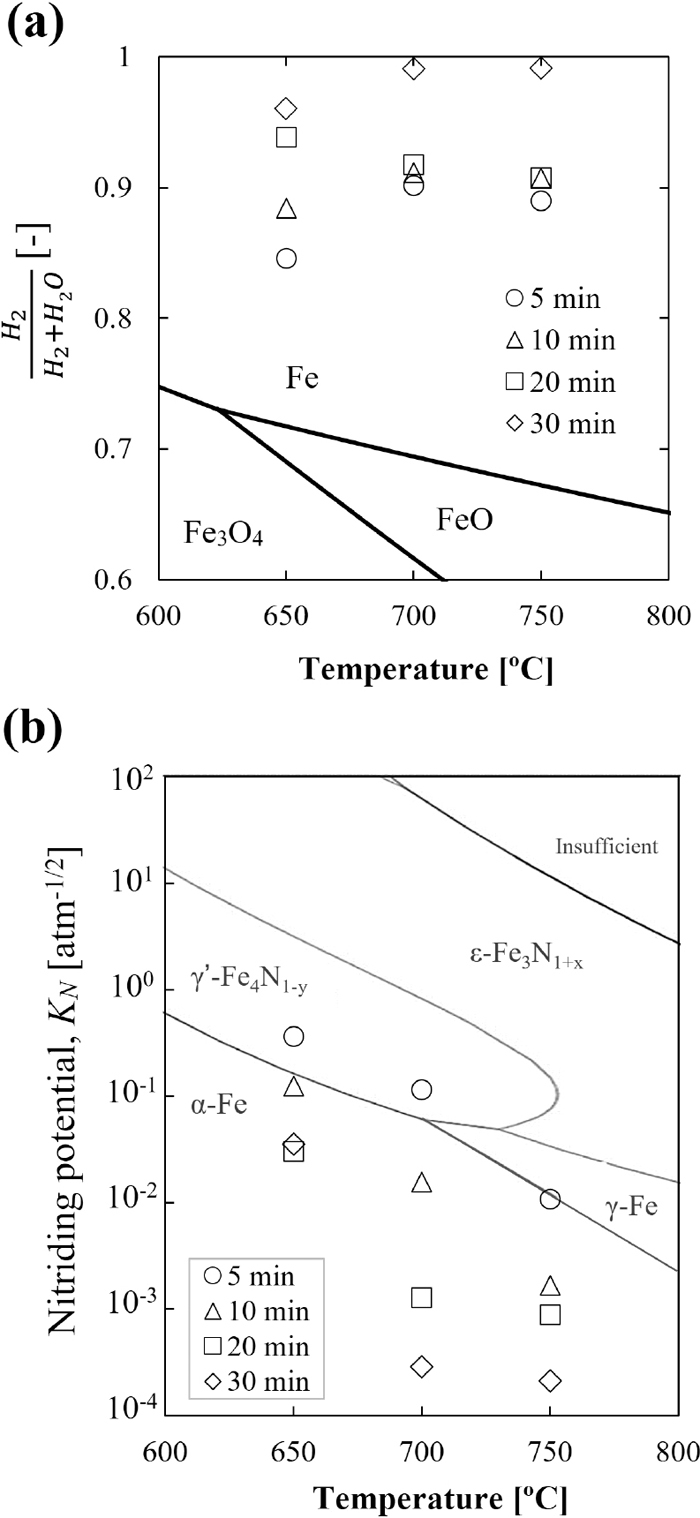
As one of the hydrogen carriers, ammonia has become one promising candidate as a reducing agent for implementing hydrogen-based ironmaking to reduce CO2 emissions. On the other hand, the abundant high combined water (CW) iron ore has recently been investigated as a raw material for ironmaking. Goethite (FeOOH), the main component of high-CW iron ore, can change to porous hematite (Fe2O3) by dehydration, enhancing its reactivity. This paper describes the fundamental study of the ore reduction behavior using ammonia as reducing agent. The effects of different ore types (i.e., high- and low-CW ores), reduction temperatures (i.e., 650, 700, and 750°C), and conditions of post-reduction treatments (i.e., quenching by NH3, fast- and slow- quenching by inert gas) on ore reduction behavior. The results reveal that the dehydrated high-CW one exhibits a higher ammonia utilization rate and is reduced faster due to the high specific surface area of the pores generated from the ore dehydration. The reduction degree of the sample increased at a higher temperature. However, in contrast, the nitriding degree decreases since the decomposition of nitrides occur highly at elevated temperatures. During quenching at temperatures lower than 700°C, the metallic Fe in the sample was nitrided in the presence of NH3. In contrast, the nitrides were easily decomposed into metallic Fe in the absence of NH3 at 700°C. This finding suggests that the quenching conditions significantly affect the generated phases. Thus, the generated phases of the reduced ore could be easily controlled in the post-reduction process.