- 著者
- Tomotaka Sasada Amartuvshin Chunag
- 出版者
- The Iron and Steel Institute of Japan
- 雑誌
- ISIJ International (ISSN:09151559)
- 巻号頁・発行日
- vol.54, no.5, pp.1017-1023, 2014-05-15 (Released:2014-06-12)
- 参考文献数
- 11
- 被引用文献数
- 3 15
The first archeological evidence in Mongolia of an iron-smelting site has been discovered at Khustyn Bulag. This site belongs to the Xiongnu age (209BCE-155CE). The Xiongnu was the first Nomadic Empire in east Eurasia. Our excavation area was quite small but contained many interesting structures (smelting furnaces, calciners or roasters of iron ore, and slag disposal pits) and artifacts (a few pieces of pottery of Xiongnu age, many clay tuyeres and slag, stone hammers and stone anvil). We could categorize the furnaces into three types, but all of them had slag pits. In addition, several clay tuyeres were used at each furnace. These characteristics of iron smelting are related not to China but to South Siberia. We also performed metallurgical and mineralogical analyses on the slag, clarified the processes of direct steel-making, roasting of iron ore (Magnetite), and found the iron mine that supplied ore to the site. We consider our research results sufficient to undertake a comparative study on Eurasia scale. This iron-smelting technology was introduced into ancient Mongolia from the West through the Steppe-Taiga area and was adjusted and locally developed (or originally), even though iron production was in conflict with the traditional nomadic life-style of the ancient Mongolians. We will continue our research, to clarify the interesting process that led nomadic society to produce political system and establish a nomadic state.
- 著者
- Yoshikazu Matsuoka Tatsuya Iwasaki Nobuo Nakada Toshihiro Tsuchiyama Setsuo Takaki
- 出版者
- The Iron and Steel Institute of Japan
- 雑誌
- ISIJ International (ISSN:09151559)
- 巻号頁・発行日
- vol.53, no.7, pp.1224-1230, 2013 (Released:2013-08-20)
- 参考文献数
- 14
- 被引用文献数
- 139 174
In order to clarify the grain size dependence of mechanical stability of austenite, deformation-induced martensitic transformation behavior was investigated on uniaxial tensile deformation in a metastable austenitic stainless steel (Fe–16%Cr–10%Ni) with the grain size controlled from 1 to 80 μm. In addition, crystallographic characteristics of deformation-induced martensite were analyzed by means of the EBSD (electron backscattering diffraction) method to discuss the variant selection rule. It was found that mechanical stability of austenite is independent of its grain size, although thermal stability of austenite is remarkably increased by grain refinement. Some special martensite variants tend to be selected in an austenite grain on the deformation-induced martensitic transformation (near single-variant transformation), and this results in the formation of a texture along tensile direction. This suggests that the most advantageous variants are selected in the deformation-induced martensitic transformation to release tensile strain and leads to the grain size independence of mechanical stability of austenite.
5 0 0 0 OA Online Prediction of Hot Metal Temperature Using Transient Model and Moving Horizon Estimation
- 著者
- Yoshinari Hashimoto Yoshitaka Sawa Manabu Kano
- 出版者
- The Iron and Steel Institute of Japan
- 雑誌
- ISIJ International (ISSN:09151559)
- 巻号頁・発行日
- vol.59, no.9, pp.1534-1544, 2019-09-15 (Released:2019-09-15)
- 参考文献数
- 27
- 被引用文献数
- 1 9
Precise control of hot metal temperature (HMT) is crucial for achieving stable operation of a blast furnace, but it is difficult due to the sluggish dynamics caused by the huge heat capacity. To cope with such difficulty, this work aims at developing a method that can predict future HMT by adopting moving horizon estimation (MHE) based on a one-dimensional transient model. MHE is useful to successively adjust model parameters so that the undesirable influence of past disturbances on the prediction is minimized. The real application result demonstrated that the root mean square error (RMSE) of HMT of eight-hour-ahead prediction was only 11.6°C. The high-performance prediction enables operators to realize the efficient operation of the blast furnace.
- 著者
- Toshihiro Tsuchiyama Takayuki Sakamoto Shohei Tanaka Takuro Masumura
- 出版者
- The Iron and Steel Institute of Japan
- 雑誌
- ISIJ International (ISSN:09151559)
- 巻号頁・発行日
- vol.60, no.12, pp.2954-2962, 2020-12-15 (Released:2020-12-16)
- 参考文献数
- 13
- 被引用文献数
- 7
Medium manganese steel (Fe-5.0%Mn-1.2%Si-0.10%C alloy) was subjected to interrupted quenching from the austenite single-phase region to a temperature between Ms and Mf followed by intercritical annealing in the ferrite and austenite dual-phase region at 923 K. As a result, a core-shell type second phase, which consisted of a fresh martensite core surrounded by a film-like retained austenite shell, was formed. The mechanism and kinetics of reversion for the interrupted-quenched specimens were analyzed with DICTRA simulation and TEM observation. With regard to the effect of the core-shell type second phase on mechanical properties, it was inferred that the fresh martensite core functioned as a hard second phase and enhanced work hardening by stress partitioning similar to DP steel, while the film-like retained austenite contributed to improved ductility due to the TRIP effect. As the interrupted quenching temperature decreased, the volume fraction of the fresh martensite core decreased, while the stability of the retained austenite shell increased. This showed potential for controlling the strength and ductility balance of medium manganese steel. A possible beneficial effect of the core-shell type second phase on the ductile fracture behavior was also discussed in terms of stress/strain relaxation at the interfaces between hard martensite and ferrite matrix.
- 著者
- Koji Sugie Akira Taniguchi
- 出版者
- 一般社団法人 日本鉄鋼協会
- 雑誌
- ISIJ International (ISSN:09151559)
- 巻号頁・発行日
- vol.51, no.3, pp.513-520, 2011-03-15 (Released:2011-03-18)
- 参考文献数
- 56
- 被引用文献数
- 4 11
The bioavailability and durability of Fe released from decarburization steelmaking slag was examined for two marine diatom species. The bioavailability of Fe released from the slag was compared with that from the reagent FeCl3·6H2O in the presence or absence of the synthetic chelator ethylenediaminetetraacetic acid, which affects Fe speciation in seawater. The duration of bioavailability was determined by the recovery of the growth rate on intermittent additions of macro-nutrients other than Fe. Abiotic reduction of bioavailable Fe from the slag in seawater was also investigated for 5 or 15 d. Thus, the bioavailability of Fe released from the slag was observed to be sufficiently high to promote the maximum growth rate; this was similar to that observed with the reagent inorganic Fe. This implies that the iron released from the slag is a dissolved ferric and/or ferrous ion/hydroxide species. In the culture media, to which the slag was added at the concentration of 20 mg L−1, the slag supplied bioavailable Fe to two diatoms for 50 d. The probable duration for which the slag was available as an Fe source was approximately 10 times longer than the reported duration in in situ iron fertilization experiments. These results indicate that continuous Fe fertilization can be achieved by a single addition of the slag, and hence, we can reduce the energy and cost of ocean fertilization and also create a resource of microalgae biofuels.
2 0 0 0 OA Optimum Water Content Estimation for Wet Granulation of Iron Ore Powders with Quicklime Binder
- 著者
- Shota Yokokawa Hideya Nakamura Tomotaka Otsu Shuji Ohsaki Satoru Watano Shohei Fujiwara Takahide Higuchi
- 出版者
- The Iron and Steel Institute of Japan
- 雑誌
- ISIJ International (ISSN:09151559)
- 巻号頁・発行日
- pp.ISIJINT-2022-498, (Released:2023-01-19)
- 参考文献数
- 31
- 被引用文献数
- 3
Wet granulation plays an important role in the processing of fine ore powder. Water content is a critical process parameter that determines the granule properties during wet granulation. However, in the ironmaking industry, various types of iron ore powder imported from different regions are blended, quicklime powder is added as a binder, and used as raw materials. Therefore, the physicochemical properties of the raw powders are not always consistent, which makes it difficult to determine the optimum water content. In this study, we present a method to determine the optimum water content using the agitation torque of wet ore powder blended with quicklime. First, we investigated the agitation torque for blending of various types and ratios of ore powders and quicklime. Two types of torque profiles were observed: a unimodal torque profile (Type I) and a torque profile with a plateau region (Type II). From the agitation torque profile, the characteristic water content (
- 著者
- Hiroyuki Uchima Masayoshi Kumagai Junzo Shimbe Akihiro Tanabe Yuta Mizuno Yusuke Onuki
- 出版者
- The Iron and Steel Institute of Japan
- 雑誌
- ISIJ International (ISSN:09151559)
- 巻号頁・発行日
- vol.62, no.5, pp.998-1003, 2022-05-15 (Released:2022-05-24)
- 参考文献数
- 38
- 被引用文献数
- 9
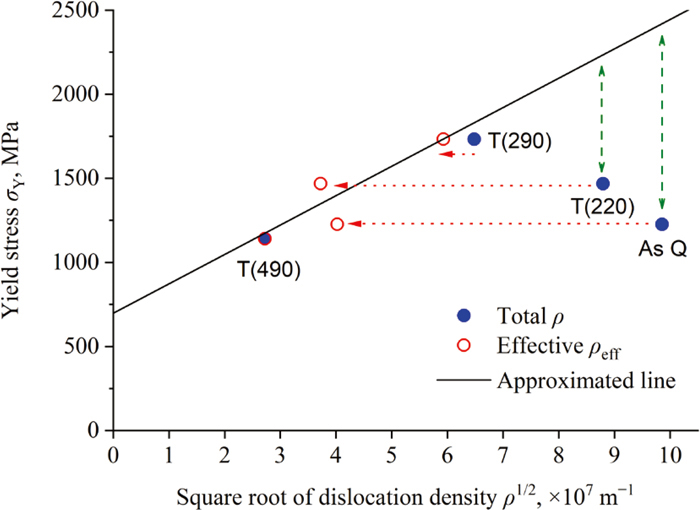
Middle-carbon martensite steels are vital materials for mechanical components and their mechanical properties have attracted significant interest. However, the decrease in the elastic limit of the as-quenched materials is one of the remaining puzzles. Herein, we quantitatively characterized the dislocation density and its structure in the as-quenched and tempered martensite steel by neutron diffraction line profile analysis and discussed their impact on the yield stress. The dislocation density in the as-quenched specimen was the highest at 9.7 × 1015 m−2, while it decreased with an increase in the tempering temperature. In addition, the component ratios of edge and screw dislocations decreased and increased, respectively, depending on the increase in the tempering temperature. The dislocation arrangement parameter (M) varied between the tempering temperatures of 220 and 290°C. Although there was a large difference between the yield stress obtained from the tensile test and that estimated from the dislocation density, the experimental results could be explained by correcting them with the inverse of M value as an index showing the effective dislocation density ratio.
2 0 0 0 OA Determining Optimum Water Content for Iron Ore Granulation using Agitation Torque of Wet Ore Powder
- 著者
- Tomotaka Otsu Hideya Nakamura Shuji Ohsaki Satoru Watano Shohei Fujiwara Takahide Higuchi
- 出版者
- The Iron and Steel Institute of Japan
- 雑誌
- ISIJ International (ISSN:09151559)
- 巻号頁・発行日
- pp.ISIJINT-2022-009, (Released:2022-03-12)
- 参考文献数
- 35
- 被引用文献数
- 5
Wet granulation of iron ore powders is a key process in ironmaking. In wet granulation, it is important to determine the optimum content of water added to the original ore powders. To determine the optimum water content, it is important to understand the saturation state in wet ore powder, which can be done by measuring the agitation torque of the wet powder. This study proposes a methodology for determining the optimum water content of various iron ore powders using the agitation torque of wet ore powders. First, measurement of the agitation torque and wet granulation of various iron ore powders were conducted. By comparing the results, it was found that the optimum water content, which was defined as the minimum water content required to diminish fine particles in the original powder, corresponded to the water content exhibiting the maximum agitation torque, regardless of the original powder. Using the agitation torque at different water contents, the saturation degree S, which is the volume ratio of water to the interparticle voids, was calculated, resulting in a range of 0.999 ≤ S ≤ 1.173 at the optimum water content. This suggests that the state between the funicular and capillary states is a suitable saturation state for the wet granulation of ore powders. Consequently, it was demonstrated that it is possible to determine the optimum water content for wet granulation of various iron ore powders based on the water content exhibiting the maximum agitation torque of wet ore powders.
- 著者
- Hiroyuki Uchima Masayoshi Kumagai Junzo Shimbe Akihiro Tanabe Yuta Mizuno Yusuke Onuki
- 出版者
- The Iron and Steel Institute of Japan
- 雑誌
- ISIJ International (ISSN:09151559)
- 巻号頁・発行日
- pp.ISIJINT-2021-443, (Released:2022-01-07)
- 参考文献数
- 38
- 被引用文献数
- 9
Middle-carbon martensite steels are vital materials for mechanical components and their mechanical properties have attracted significant interest. However, the decrease in the elastic limit of the as-quenched materials is one of the remaining puzzles. Herein, we quantitatively characterized the dislocation density and its structure in the as-quenched and tempered martensite steel by neutron diffraction line profile analysis and discussed their impact on the yield stress. The dislocation density in the as-quenched specimen was the highest at 9.7 × 1015 m-2, while it decreased with an increase in the tempering temperature. In addition, the component ratios of edge and screw dislocations decreased and increased, respectively, depending on the increase in the tempering temperature. The dislocation arrangement parameter (M) varied between the tempering temperatures of 220 and 290°C. Although there was a large difference between the yield stress obtained from the tensile test and that estimated from the dislocation density, the experimental results could be explained by correcting them with the inverse of M value as an index showing the effective dislocation density ratio.
2 0 0 0 OA Comparison of Microstructure and Hardness between High-carbon and High-nitrogen Martensites
- 著者
- Toshihiro Tsuchiyama Kurato Inoue Katsutoshi Hyodo Daichi Akama Nobuo Nakada Setsuo Takaki Tamotsu Koyano
- 出版者
- The Iron and Steel Institute of Japan
- 雑誌
- ISIJ International (ISSN:09151559)
- 巻号頁・発行日
- vol.59, no.1, pp.161-168, 2019-01-15 (Released:2019-01-17)
- 参考文献数
- 37
- 被引用文献数
- 9 16
The microstructure and hardness of martensite in Fe–C and Fe–N alloys with up to 7.5 at% contents of carbon and nitrogen, respectively, were compared. Their difference in hardness was discussed based on four strengthening mechanisms. The martensitic structures of Fe–C and Fe–N alloys with equal contents of carbon and nitrogen, respectively, were nearly identical, except for the amount of retained austenite. Furthermore, Fe–C alloy was considerably harder than Fe–N alloy. This discrepancy gradually increased with carbon and nitrogen contents. The enhanced hardness of Fe–C alloy martensite was attributed to its higher dislocation density and the stronger pinning force of interstitial carbon atoms on dislocations.
- 著者
- Harshad Kumar Dharamshi Hansraj Bhadeshia
- 出版者
- The Iron and Steel Institute of Japan
- 雑誌
- ISIJ International (ISSN:09151559)
- 巻号頁・発行日
- vol.56, no.1, pp.24-36, 2016-01-15 (Released:2016-01-29)
- 参考文献数
- 169
- 被引用文献数
- 118 400
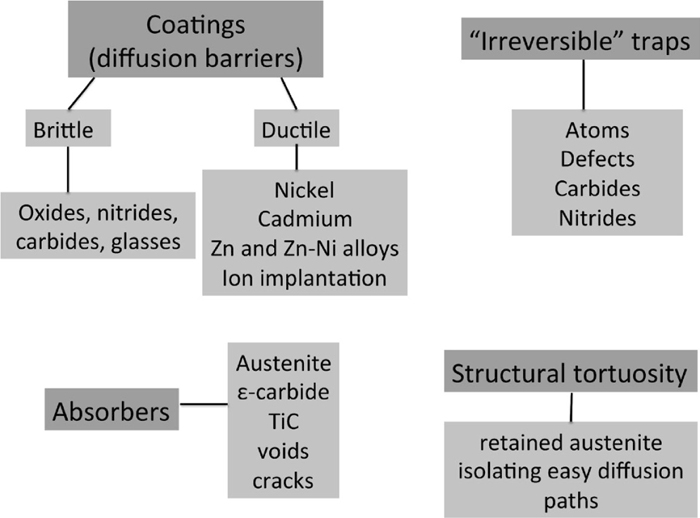
The essential facts about the nature of the hydrogen embrittlement of steels have now been known for 140 years. It is diffusible hydrogen that is harmful to the toughness of iron. It follows, therefore, that the harmful influence of diffusible hydrogen can be mitigated by preventing its entry into steel or by rendering it immobile once it penetrates the material. This review deals with the methods that might be implemented to design steels and components that resist hydrogen embrittlement by reducing the intake of hydrogen or rendering it innocuous when it does penetrate the steel.
- 著者
- Jin Jia Shang-lei Yang Wei-yuan Ni Jian-ying Bai Yang-shenglan Lin
- 出版者
- 一般社団法人 日本鉄鋼協会
- 雑誌
- ISIJ International (ISSN:09151559)
- 巻号頁・発行日
- vol.55, no.1, pp.338-338, 2015 (Released:2015-02-06)
- 著者
- Hiroshi Tanii Tadahiro Inazumi Keiichi Terashima
- 出版者
- The Iron and Steel Institute of Japan
- 雑誌
- ISIJ International (ISSN:09151559)
- 巻号頁・発行日
- vol.54, no.5, pp.1044-1050, 2014-05-15 (Released:2014-06-12)
- 参考文献数
- 9
- 被引用文献数
- 7 7
This study is to investigate and clarify the details about mineralogical characteristics of iron sand used for Japanese classical iron-making furnace “Tatara”, that produces different kinds of iron product, natural steel and cast iron. Though the product difference is known experientially to be attributed to the difference of iron sand kind “Masa” and “Akome”, scientific reason is vague and their characterization is necessary. Specimen was prepared by separating the particles with the similar character by sieving and magnetic separation. Chemical analysis, XRD, mineral texture, EPMA, chromaticity and diffusive reflectance, Raman spectroscopy and specific gravity clarified that main difference between Masa and Akome is in the composition of particles mixed with different mineral characters in term of solid solution TiO2% in iron oxide and quantity of hematite that is weathered magnetite. CO gas reduction test showed that such mineral characters affect the initial temperature of reduction and mineral kinds formed on the way of reduction.
- 著者
- Yasuhiko Miyoshi
- 出版者
- The Iron and Steel Institute of Japan
- 雑誌
- ISIJ International (ISSN:09151559)
- 巻号頁・発行日
- vol.31, no.1, pp.1-10, 1991-01-15 (Released:2007-05-31)
- 参考文献数
- 37
- 被引用文献数
- 15 19
The history of precoated steel usage as car body materials in Japan is reviewed first. Japanese steelmakers developed galvannealed steel, duplex Zn–Fe coated steel, Zn–Ni coated steel and organic composite coated steel. All of them have been widely used. Recently, electrolytically Fe–Zn plated galvannealed steel and 1μm thick organic painted Zn–Ni electroplated steel have been applied. They have not only excellent corrosion resistance, but also good paintability, formability and weldability. Car body corrosion is classified into cosmetic corrosion and perforation. Newly clarified mechanisms for these two are explained. As for current research subjects, the development of inorganic and organic dispersion coating, Zn–Mn plating, galvannealed steel by vapor deposition, and vapor phase deposited Zn–Mg coating are described. Surface roughness control, application to vibration damping sheet, adhesive bonding, and the adaptation to lightweight cars are also important subjects to be studied now.
- 著者
- Itsuki Iwamoto Ade Kurniawan Hiroki Hasegawa Yoshiaki Kashiwaya Takahiro Nomura Tomohiro Akiyama
- 出版者
- The Iron and Steel Institute of Japan
- 雑誌
- ISIJ International (ISSN:09151559)
- 巻号頁・発行日
- vol.62, no.12, pp.2483-2490, 2022-12-15 (Released:2022-12-15)
- 参考文献数
- 38
- 被引用文献数
- 2
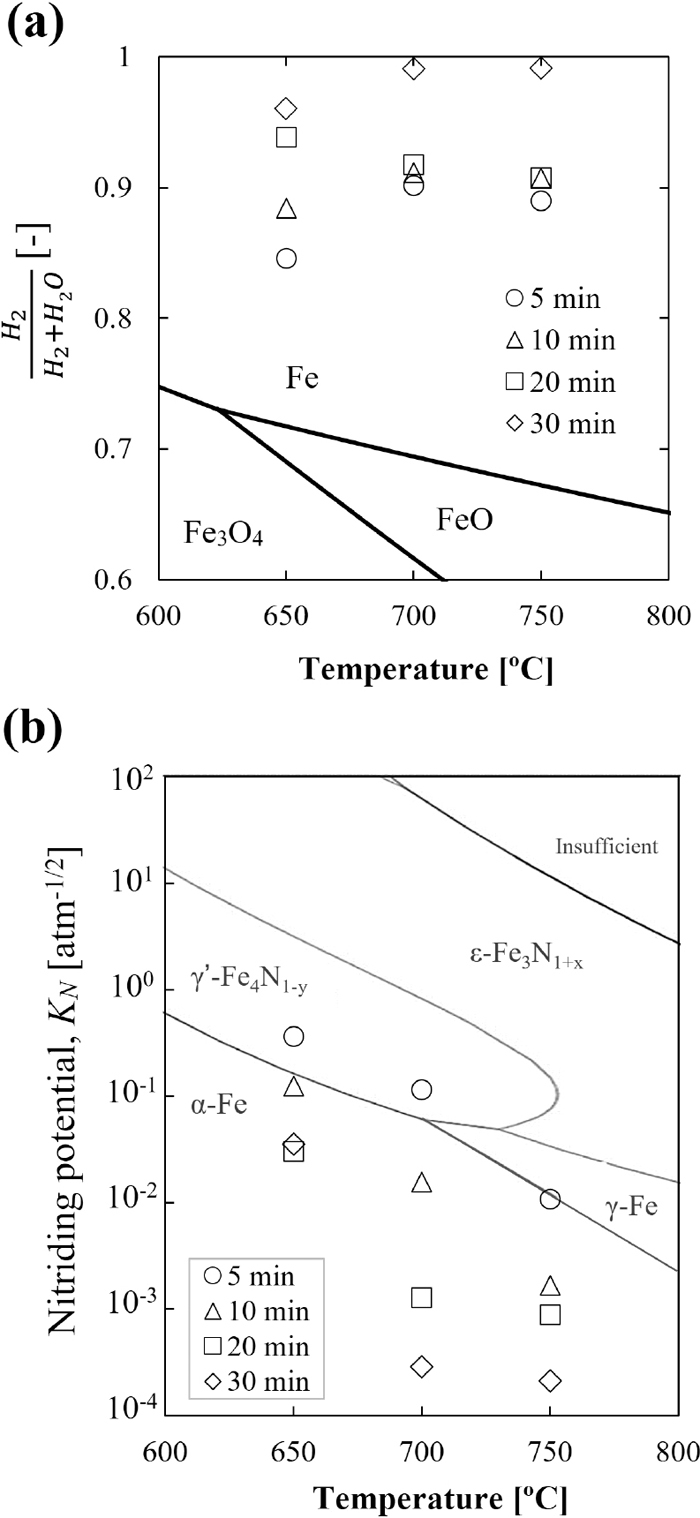
As one of the hydrogen carriers, ammonia has become one promising candidate as a reducing agent for implementing hydrogen-based ironmaking to reduce CO2 emissions. On the other hand, the abundant high combined water (CW) iron ore has recently been investigated as a raw material for ironmaking. Goethite (FeOOH), the main component of high-CW iron ore, can change to porous hematite (Fe2O3) by dehydration, enhancing its reactivity. This paper describes the fundamental study of the ore reduction behavior using ammonia as reducing agent. The effects of different ore types (i.e., high- and low-CW ores), reduction temperatures (i.e., 650, 700, and 750°C), and conditions of post-reduction treatments (i.e., quenching by NH3, fast- and slow- quenching by inert gas) on ore reduction behavior. The results reveal that the dehydrated high-CW one exhibits a higher ammonia utilization rate and is reduced faster due to the high specific surface area of the pores generated from the ore dehydration. The reduction degree of the sample increased at a higher temperature. However, in contrast, the nitriding degree decreases since the decomposition of nitrides occur highly at elevated temperatures. During quenching at temperatures lower than 700°C, the metallic Fe in the sample was nitrided in the presence of NH3. In contrast, the nitrides were easily decomposed into metallic Fe in the absence of NH3 at 700°C. This finding suggests that the quenching conditions significantly affect the generated phases. Thus, the generated phases of the reduced ore could be easily controlled in the post-reduction process.
1 0 0 0 OA Natural Convection on Dendrite Morphology: A High–performance Phase–field Lattice Boltzmann Study
- 著者
- Tomohiro Takaki Shinji Sakane Takayuki Aoki
- 出版者
- The Iron and Steel Institute of Japan
- 雑誌
- ISIJ International (ISSN:09151559)
- 巻号頁・発行日
- vol.63, no.1, pp.83-90, 2023-01-15 (Released:2023-01-21)
- 参考文献数
- 50
- 被引用文献数
- 3
Numerical study on the effect of liquid flow on three-dimensional dendrite growth is still a challenging topic. Herein, high-performance phase–field lattice Boltzmann (PF-LB) simulations were performed to investigate the effect of natural convection on dendrite morphology and the possibility for causing fragmentation. Parallel computing in multiple graphics processing units (GPUs) with dynamic load balancing for the block-structured adaptive mesh refinement (AMR) scheme (parallel-GPU AMR) was applied to the PF-LB simulations as a high-performance computing tool in a GPU supercomputer. Parallel-GPU AMR PF-LB simulations showed that the growth of dendrites with natural convection in two and three dimensions were quite different. The dendrite tip velocity increased in the following order: upward buoyancy, no gravity, and downward buoyancy. Downward and upward buoyancy enhanced and restricted the growth of the secondary arms, respectively. The root size of the secondary arms growing from the bottom was drastically affected by the flow direction. However, the dendrite fragmentations were not observed in the present simulations.
- 著者
- Takuya Kiyozumi Shinji Kudo Aska Mori Riku Mizoguchi Atsushi Tahara Shusaku Asano Jun-ichiro Hayashi
- 出版者
- The Iron and Steel Institute of Japan
- 雑誌
- ISIJ International (ISSN:09151559)
- 巻号頁・発行日
- vol.62, no.12, pp.2476-2482, 2022-12-15 (Released:2022-12-15)
- 参考文献数
- 26
Oxalic acid is an attractive chemical platform potentially available from CO2 due to its established applications and chemical characteristics enabling it to serve as a mediator in hydrometallurgy including iron-making. However, a method for synthesizing oxalic acid from CO2 has yet to be established. In the present work, the formation of oxalate scaffold during heating of cesium carbonate (Cs2CO3) in the presence of CO2 and H2 as reactants was experimentally investigated with a particular focus on the influence of supporting Cs2CO3 over porous materials. Among the support materials examined, activated carbon (AC) had a notable effect in improving the reaction rate and yield of total carboxylates (formate and oxalate) during experiments with an autoclave. An important problem was the dominant presence of formate, the intermediate between carbonate and oxalate, accounting for over 90% of the carboxylates. Changing the reaction conditions, including temperature, reaction time, partial pressure of gas components, and amount of loaded Cs2CO3, did not alter the situation. Alternatively, re-heating of the formate-rich salts over AC under CO and CO2 enhanced the oxalate fraction while maintaining the total carboxylates yield. Benefiting from the employment of support material, the two-step conversion was carried out using a gas-flow type reactor with a packed bed of Cs2CO3 supported over AC. In this reaction system, because water, acting as a promoter, was absent, the total carboxylates yield was lower than that in the autoclave, while the oxalate fraction was higher, being 71.8% with a yield of 43.2% on a Cs2CO3-carbon basis.
- 著者
- Keita Tanahashi Yusei Omura Hidekazu Naya Yuji Kunisada Norihito Sakaguchi Ade Kurniawan Takahiro Nomura
- 出版者
- The Iron and Steel Institute of Japan
- 雑誌
- ISIJ International (ISSN:09151559)
- 巻号頁・発行日
- vol.62, no.12, pp.2578-2586, 2022-12-15 (Released:2022-12-15)
- 参考文献数
- 30
- 被引用文献数
- 1
High-temperature pressure swing adsorption (HT-PSA) is a promising energy-saving approach for oxygen production from air. Ca2AlMnO5+δ, a Brownmillerite-type perovskite, is a promising sorbent for HT-PSA because of its remarkably high oxygen storage capacity (up to 3.3 wt%). In this study, we investigated the redox thermodynamics of Ca2AlMnO5+δ by pressure–composition–temperature (PCT) measurements and investigated the HT-PSA performance of Ca2AlMnO5+δ pellets in a 100 g-scale packed-bed-type reactor. PCT measurements revealed that Ca2AlMnO5+δ can reversibly separate 2.2 wt% of oxygen per cycle under equilibrium conditions between ambient oxygen partial pressure and 5×10−4 MPa at 525°C. However, in a 5 min switching HT-PSA test, Ca2AlMnO5+δ pellets were able to reversibly separate less than 1 wt% oxygen per cycle, which is significantly lower than that estimated from the thermodynamic properties of Ca2AlMnO5+δ. On the other hand, the exothermic oxygen storage and endothermic oxygen release reactions cause significant temperature variation of the packed bed. This study clarifies that, in order to increase the energy efficiency of oxygen separation by HT-PSA, there is a need to compensate for the heat of reaction, which changes the reactor temperature in a direction that interferes with the reaction.
- 著者
- Yushi Takenouchi Shuhei Wada Takuro Masumura Toshihiro Tsuchiyama Hiroshi Okano Shusaku Takagi
- 出版者
- The Iron and Steel Institute of Japan
- 雑誌
- ISIJ International (ISSN:09151559)
- 巻号頁・発行日
- vol.62, no.10, pp.2000-2007, 2022-10-15 (Released:2022-10-19)
- 参考文献数
- 23
- 被引用文献数
- 2
Stress relaxation tests were conducted in the elastic region of an ultralow carbon martensitic steel (Fe–18%Ni alloy) to quantitatively analyze the effect of mobile dislocations on the low elastic limit of the steel. The elastic limit of the as-quenched material was measured at 255 MPa, although its tensile strength was as high as 720 MPa. The stress relaxation tests, which were performed at 255 MPa, revealed a remarkable stress reduction due to the movement of the mobile dislocations present in the as-quenched material. The total dislocation density barely changed during the test, while the distribution parameter (M-value) decreased significantly, indicating that the mobile dislocations exhibited stable arrangements. The 5% cold rolling before the relaxation tests suppressed the relaxation and simultaneously increased the elastic limit to a maximum, 435 MPa. By estimating the mobile dislocation density by relating the stress reduction in the stress relaxation tests to the distance of the dislocation movement evaluated via transmission electron microscopy (TEM) observations, it was estimated that the mobile dislocation density of the 5%-cold-rolled material was lowered to ~1/10 of that of the as-quenched material.
- 著者
- Yuki Tampa Kosuke Takagi Shohei Ueki Motoki Ohta Yoji Mine Kazuki Takashima
- 出版者
- The Iron and Steel Institute of Japan
- 雑誌
- ISIJ International (ISSN:09151559)
- 巻号頁・発行日
- vol.62, no.8, pp.1741-1749, 2022-08-15 (Released:2022-08-18)
- 参考文献数
- 27
- 被引用文献数
- 3
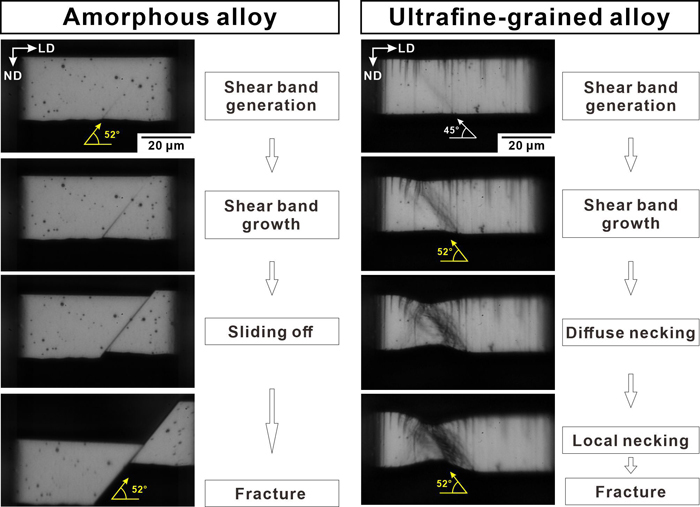
Micro-tensile tests were employed to clarify the post-plastic-instability behavior in the shear fractures of specimens with the dimensions of 18×25×50 µm3 made from iron-based amorphous (AM) and ultrafine-grained (UFG) alloys. The AM specimen yielded by localized shear bands with an inclination angle of ~52° with respect to the loading axis, followed by sliding off almost throughout the entire specimen thickness. Micro-tensile and micro-shearing tests revealed that the Mohr failure envelope of the AM specimens could be described by a quadratic equation rather than a linear equation. Therefore, the sliding-off process is assisted by the applied normal stress, which suggests that it is caused by free-volume coalescence. For the UFG specimen, yielding set in by shear band formation with an inclination angle of ~45° with respect to the loading axis, following the Tresca criterion. Necking after shear band diffusion formed a triaxial stress state, which resulted in a final shear fracture plane via void coalescence in the UFG specimens. Voids formed along the intersection of the primary shear bands with secondary shear bands during the necking process. This indicates that the deviation of the shear fracture plane in the UFG specimen was determined by the strain development process. A comparison of the post-plastic-instability behavior between the AM and UFG specimens suggests that the external control of triaxial stress conditions is key to improving the formability of AM specimens.