6 0 0 0 OA Dosing Optimization of Ampicillin-Sulbactam Based on Cystatin C in Elderly Patients with Pneumonia
- 著者
- Kayoko Matsubara Kazuaki Matsumoto Yuta Yokoyama Erika Watanabe Yuki Enoki Akari Shigemi Kazuro Ikawa Hideyuki Terazono Norifumi Morikawa Tamao Ohshige Yasuo Takeda
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.44, no.5, pp.732-736, 2021-05-01 (Released:2021-05-01)
- 参考文献数
- 27
- 被引用文献数
- 3
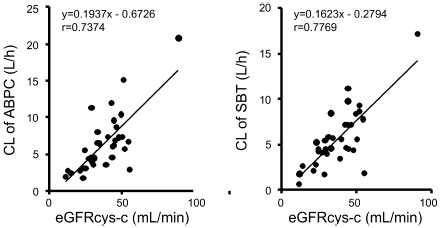
Ampicillin-sulbactam is a first-line therapy for pneumonia and is mainly excreted by the kidney. It is important to optimize the dose and dosing interval of ampicillin-sulbactam because in patients with decreased renal function and low skeletal muscle mass, such as the elderly, excess drug may burden renal function. In this study, we evaluated indices of renal function and optimized the dose and dosing interval of ampicillin-sulbactam based on pharmacokinetics (PK) and pharmacodynamics theory in elderly patients. The serum concentrations of ampicillin and sulbactam were measured by HPLC, and PK parameters were calculated. Correlations between the clearance of ampicillin or sulbactam and renal function were evaluated, and dosing optimization was calculated based on PK parameters. The PK parameters of ampicillin were CL = 6.5 ± 4.0 L/h, Vd = 19.3 ± 0.2 L, Ke = 0.4 ± 0.2, and t1/2 = 2.7 ± 1.6 h. The most correlated renal function index was estimated glomerular filtration rate (eGFRcys-c) calculated by serum cystatin-c (r = 0.7374, correlation formula; CL of ampicillin = 0.1937 × eGFRcys-c−0.6726). Based on this formula, we calculated the clearance of ampicillin and developed dosing regimens for the elderly. Serum cystatin-c concentration is an ideal index to optimize ampicillin-sulbactam antimicrobial therapy in elderly patients with pneumonia.
2 0 0 0 OA Evaluation of the Expression Time of Ganciclovir-Induced Adverse Events Using JADER and FAERS
- 著者
- Go Ando Kazuaki Taguchi Yuki Enoki Yuta Yokoyama Junko Kizu Kazuaki Matsumoto
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.42, no.11, pp.1799-1804, 2019-11-01 (Released:2019-11-01)
- 参考文献数
- 20
- 被引用文献数
- 22
Investigation of the occurrence time of adverse drug reactions helps to prevent the development and aggravation of adverse reactions, but the expression time of ganciclovir-induced adverse events has not been elucidated. In this study, using databases of spontaneous adverse event reports, the Japanese Adverse Drug Event Report database (JADER) and the U.S. Food and Drug Administration (FDA)’s Adverse Event Reporting System (FAERS), the incidence of adverse reactions due to ganciclovir and their expression time were analyzed. As a result of calculation of the reporting odds ratio (ROR) and 95% confidence interval for individual main adverse reactions of ganciclovir (cytopenia, leukopenia, thrombocytopenia, liver damage, and acute renal failure), a signal was detected for all adverse reactions in both databases, except for liver damage in JADER. Furthermore, the Weibull distribution was performed for the analysis of onset time of each ganciclovir-induced adverse event. The results of Weibull parameter α and β values of each adverse event in both JADER and FAERS suggested that most adverse events occurred within 30 d and classified into the early failure type, except that thrombocytopenia and acute renal failure in JADER classified into the random failure type. Based on these findings, it concluded that the paying attention to signs of each ganciclovir-induced adverse event is required from the early phase after ganciclovir administration. However, in FAERS, development after a long-term course also accounted for 11%, suggesting that long-term periodic monitoring of adverse reactions would be also required.
- 著者
- Kengo Hanaya Kazuaki Matsumoto Yuta Yokoyama Junko Kizu Mitsuru Shoji Takeshi Sugai
- 出版者
- 公益社団法人日本薬学会
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.65, no.2, pp.194-199, 2017-02-01 (Released:2017-02-01)
- 参考文献数
- 14
- 被引用文献数
- 1
Linezolid (1) is an oxazolidinone antibiotic that is partially metabolized in vivo via ring cleavage of its morpholine moiety to mainly form two metabolites, PNU-142300 (2) and PNU-142586 (3). It is supposed that accumulation of 2 and 3 in patients with renal insufficiency may cause thrombocytopenia, one of the adverse effects of linezolid. However, the poor availability of 2 and 3 has hindered further investigation of the clinical significance of the accumulation of these metabolites. In this paper, we synthesized metabolites 2 and 3 via a common synthetic intermediate, 4; this will encourage further exploration of events related to these metabolites and lead to improved clinical use of linezolid.