6 0 0 0 OA Dosing Optimization of Ampicillin-Sulbactam Based on Cystatin C in Elderly Patients with Pneumonia
- 著者
- Kayoko Matsubara Kazuaki Matsumoto Yuta Yokoyama Erika Watanabe Yuki Enoki Akari Shigemi Kazuro Ikawa Hideyuki Terazono Norifumi Morikawa Tamao Ohshige Yasuo Takeda
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.44, no.5, pp.732-736, 2021-05-01 (Released:2021-05-01)
- 参考文献数
- 27
- 被引用文献数
- 3
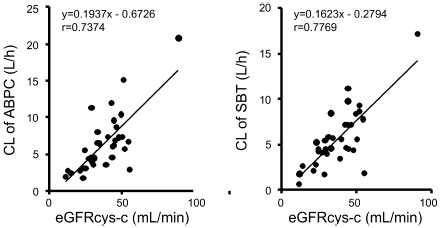
Ampicillin-sulbactam is a first-line therapy for pneumonia and is mainly excreted by the kidney. It is important to optimize the dose and dosing interval of ampicillin-sulbactam because in patients with decreased renal function and low skeletal muscle mass, such as the elderly, excess drug may burden renal function. In this study, we evaluated indices of renal function and optimized the dose and dosing interval of ampicillin-sulbactam based on pharmacokinetics (PK) and pharmacodynamics theory in elderly patients. The serum concentrations of ampicillin and sulbactam were measured by HPLC, and PK parameters were calculated. Correlations between the clearance of ampicillin or sulbactam and renal function were evaluated, and dosing optimization was calculated based on PK parameters. The PK parameters of ampicillin were CL = 6.5 ± 4.0 L/h, Vd = 19.3 ± 0.2 L, Ke = 0.4 ± 0.2, and t1/2 = 2.7 ± 1.6 h. The most correlated renal function index was estimated glomerular filtration rate (eGFRcys-c) calculated by serum cystatin-c (r = 0.7374, correlation formula; CL of ampicillin = 0.1937 × eGFRcys-c−0.6726). Based on this formula, we calculated the clearance of ampicillin and developed dosing regimens for the elderly. Serum cystatin-c concentration is an ideal index to optimize ampicillin-sulbactam antimicrobial therapy in elderly patients with pneumonia.
3 0 0 0 OA 近世中後期出羽国宝幢寺における寺役人の職分・身分 : 近世寺院領主の統治機構とその特質
- 著者
- 松本 和明 Kazuaki Matsumoto
- 雑誌
- 人文論究 (ISSN:02866773)
- 巻号頁・発行日
- vol.57, no.4, pp.20-38, 2008-02-28
- 著者
- Kazuaki Matsumoto Masaru Samura Sho Tashiro Shino Shishido Reika Saiki Wataru Takemura Kana Misawa Xiaoxi Liu Yuki Enoki Kazuaki Taguchi
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.45, no.7, pp.824-833, 2022-07-01 (Released:2022-07-01)
- 参考文献数
- 69
- 被引用文献数
- 6
The target therapeutic ranges of vancomycin, teicoplanin, and arbekacin have been determined, and therapeutic drug monitoring (TDM) is performed in clinical practice. However, TDM is not obligatory for daptomycin, linezolid, or tedizolid. In this study, we examined whether TDM will be necessary for these 3 drugs in the future. There was no significant difference in therapeutic effects on acute bacterial skin and skin structure infection between linezolid and tedizolid by meta-analysis. Concerning the therapeutic effects on pneumonia, the rate of effectiveness after treatment with tedizolid was significantly lower than with linezolid. With respect to safety, the incidences of gastrointestinal adverse events and blood/lymphatic system disorders related to tedizolid were significantly lower than those related to linezolid. Linezolid exhibits potent therapeutic effects on pneumonia, but the appearance of adverse reactions is indicated as a problem. There was a dose-dependent decrease in the platelet count, and the target trough concentration (Ctrough) was estimated to be 4–6 or 2–7 µg/mL in accordance with the patient’s condition. The efficacy of linezolid may be obtained while minimizing the appearance of adverse reactions by performing TDM. The target therapeutic range of tedizolid cannot be achieved in immunocompromised or severe patients. Therefore, we concluded that TDM was unnecessary, considering step-down therapy with oral drugs, use in non-severe patients, and high-level safety. Concerning daptomycin, high-dose administration is necessary to achieve an area under the curve (AUC) of ≥666 as an index of efficacy. To secure its safety, Ctrough (<20 µg/mL) monitoring is important. Therefore, TDM is necessary.
2 0 0 0 OA Evaluation of the Expression Time of Ganciclovir-Induced Adverse Events Using JADER and FAERS
- 著者
- Go Ando Kazuaki Taguchi Yuki Enoki Yuta Yokoyama Junko Kizu Kazuaki Matsumoto
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.42, no.11, pp.1799-1804, 2019-11-01 (Released:2019-11-01)
- 参考文献数
- 20
- 被引用文献数
- 22
Investigation of the occurrence time of adverse drug reactions helps to prevent the development and aggravation of adverse reactions, but the expression time of ganciclovir-induced adverse events has not been elucidated. In this study, using databases of spontaneous adverse event reports, the Japanese Adverse Drug Event Report database (JADER) and the U.S. Food and Drug Administration (FDA)’s Adverse Event Reporting System (FAERS), the incidence of adverse reactions due to ganciclovir and their expression time were analyzed. As a result of calculation of the reporting odds ratio (ROR) and 95% confidence interval for individual main adverse reactions of ganciclovir (cytopenia, leukopenia, thrombocytopenia, liver damage, and acute renal failure), a signal was detected for all adverse reactions in both databases, except for liver damage in JADER. Furthermore, the Weibull distribution was performed for the analysis of onset time of each ganciclovir-induced adverse event. The results of Weibull parameter α and β values of each adverse event in both JADER and FAERS suggested that most adverse events occurred within 30 d and classified into the early failure type, except that thrombocytopenia and acute renal failure in JADER classified into the random failure type. Based on these findings, it concluded that the paying attention to signs of each ganciclovir-induced adverse event is required from the early phase after ganciclovir administration. However, in FAERS, development after a long-term course also accounted for 11%, suggesting that long-term periodic monitoring of adverse reactions would be also required.
- 著者
- Kazuaki Matsumoto Akari Shigemi Kazuro Ikawa Naoko Kanazawa Yuko Fujisaki Norifumi Morikawa Yasuo Takeda
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.38, no.2, pp.235-238, 2015-02-01 (Released:2015-02-01)
- 参考文献数
- 17
- 被引用文献数
- 5 22
Ganciclovir is a nucleoside guanosine analogue that exhibits therapeutic activity against human cytomegalovirus infection, and is primarily excreted via glomerular filtration and active tubular secretion. The adverse effects induced by ganciclovir therapy are generally of a hematological nature and include thrombocytopenia and leukopenia. Low marrow cellularity and elevated serum creatinine have been identified as risk factors for ganciclovir-induced neutropenia. However, the risk factors for thrombocytopenia have yet to be determined. Therefore, this study investigated patients administered ganciclovir to determine the risk factors for thrombocytopenia and leukopenia. Thrombocytopenia occurred in 41 of these patients (30.6%). Multivariate logistic regression analysis identified three independent risk factors for thrombocytopenia: cancer chemotherapy (odds ratio (OR)=3.1), creatinine clearance (<20 mL/min) (OR=12.8), and the ganciclovir dose (≥12 mg/kg/d) (OR=15.1). Leukopenia occurred in 36 patients (28.6%), and white blood cell count (<6000 cells/mm3) (OR=3.7) and the ganciclovir dose (≥12 mg/kg/d) (OR=7.8) were identified as risk factors. These results demonstrated that several factors influenced the occurrence of ganciclovir-induced thrombocytopenia and leukopenia, and suggest that special attention should be paid to patients receiving cancer chemotherapy with a low creatinine clearance (<20 mL/min) and high dose (≥12 mg/kg/d) in order to avoid ganciclovir-induced thrombocytopenia.
- 著者
- Kengo Hanaya Kazuaki Matsumoto Yuta Yokoyama Junko Kizu Mitsuru Shoji Takeshi Sugai
- 出版者
- 公益社団法人日本薬学会
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.65, no.2, pp.194-199, 2017-02-01 (Released:2017-02-01)
- 参考文献数
- 14
- 被引用文献数
- 1
Linezolid (1) is an oxazolidinone antibiotic that is partially metabolized in vivo via ring cleavage of its morpholine moiety to mainly form two metabolites, PNU-142300 (2) and PNU-142586 (3). It is supposed that accumulation of 2 and 3 in patients with renal insufficiency may cause thrombocytopenia, one of the adverse effects of linezolid. However, the poor availability of 2 and 3 has hindered further investigation of the clinical significance of the accumulation of these metabolites. In this paper, we synthesized metabolites 2 and 3 via a common synthetic intermediate, 4; this will encourage further exploration of events related to these metabolites and lead to improved clinical use of linezolid.