7 0 0 0 OA 金属の表面物性と摩擦
- 著者
- 玉井 康勝 西山 誼行
- 出版者
- 一般社団法人 表面技術協会
- 雑誌
- 金属表面技術 (ISSN:00260614)
- 巻号頁・発行日
- vol.13, no.12, pp.497-502, 1962-12-20 (Released:2009-10-30)
- 参考文献数
- 21
- 被引用文献数
- 1
4 0 0 0 OA 表面處理としてみた電解コンデンサの諸問題
- 著者
- 佐藤 一郎
- 出版者
- 一般社団法人 表面技術協会
- 雑誌
- 金属表面技術 (ISSN:00260614)
- 巻号頁・発行日
- vol.4, no.3, pp.120-124, 1953-07-31 (Released:2009-10-30)
- 参考文献数
- 41
3 0 0 0 OA ホウ砂とカーボランダムの熱浴による軟鋼表面の浸ホウ硬化処理の研究
- 著者
- 吉岡 正三 山本 久 熊谷 雅晴
- 出版者
- 一般社団法人 表面技術協会
- 雑誌
- 金属表面技術 (ISSN:00260614)
- 巻号頁・発行日
- vol.21, no.5, pp.272-276, 1970-05-01 (Released:2009-10-30)
- 参考文献数
- 5
- 被引用文献数
- 3 1
A simple and economical process for boriding metal surfaces was found. The process was only to immerse the metal in a hot salt bath containing borax and carborundum, where no special atmosphere was needed.Optimum conditions found for boriding of mild steel were to dip the steel in a bath containing 60-50wt% of borax and 40-50wt% of carborundum at 1, 000°C for 6hrs. The boride layer produced by this process was composed of Fe2B, which was 170μ in thickness, having about 1, 200 of Vickers hardness.The mild steel lying beneath the boride layer was also observed hardened. The results of chemical analysis and electron probe microanalysis showed that the hardening of the interior mild steel was attributed to the boron and carbon penetrating through the boride layer.
3 0 0 0 OA 硫酸銅溶液中での鉄に対する銅の化学置換メッキ
- 著者
- 尾形 幹夫
- 出版者
- 一般社団法人 表面技術協会
- 雑誌
- 金属表面技術 (ISSN:00260614)
- 巻号頁・発行日
- vol.26, no.12, pp.592-596, 1975-12-01 (Released:2009-10-30)
- 参考文献数
- 5
- 被引用文献数
- 1
Experiments were carried out to find the mechanism of galvanic displacement: Fe+Cu2+→Fe2++Cu. When iron is immersed in a concentrated copper sulfate solution, e.g., 0.5M, a poorly adherent copper is deposited. In this case FeSO4 or Fe2O3 coprecipitated with the copper because the concentration of Fe2+ near the interface increased rapidly immediately after the immersion. However, atyell adherent copper was deposited by immersion in an acidic dilute copper sulfate solution, e.g., 0.01M, pH 1.5. In this case the rate of copper deposition, namely, the rate of formation of Fe2+ was slow, so that the Fe2+ was permitted to diffuse into the bulk of the solution without precipitating, which was the case of well adherent copper plate. The formulation of immersion plating of copper on iron was: CuSO4 0.0025-0.03M, pH<2.5, room temperature, immersion time 0.5-15min. The copper plating applied under these conditions was adherent to iron, hence copper could be electroplated on it from the ordinary pyrophosphate baths.
2 0 0 0 OA 肥後象眼について
- 著者
- 堀 一夫
- 出版者
- 一般社団法人 表面技術協会
- 雑誌
- 金属表面技術 (ISSN:00260614)
- 巻号頁・発行日
- vol.37, no.6, pp.289-294, 1986-06-01 (Released:2009-10-30)
- 参考文献数
- 3
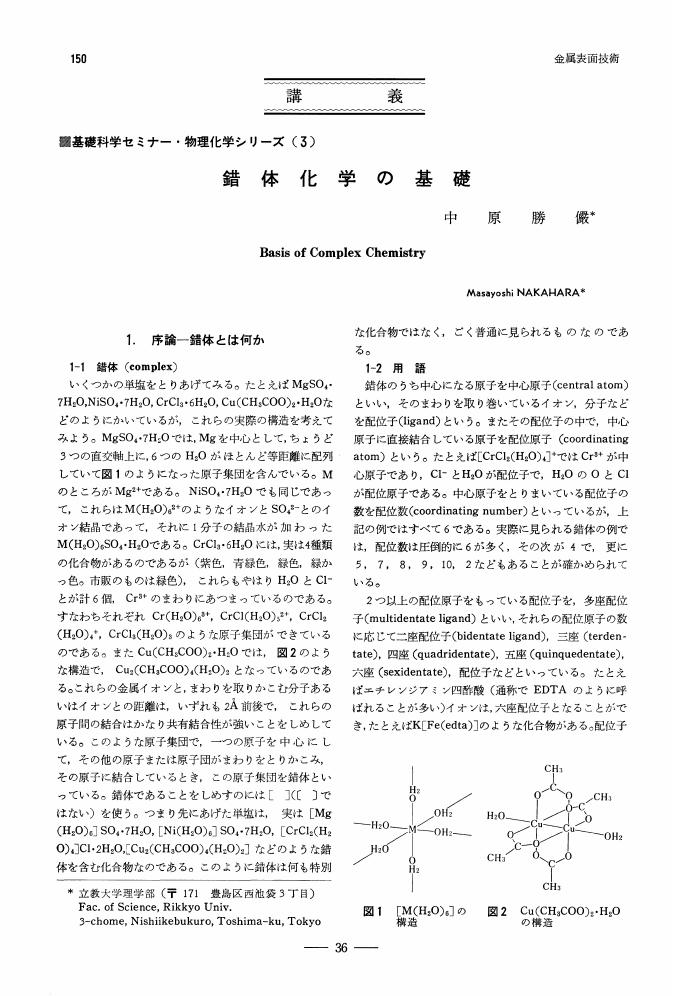
2 0 0 0 OA 錯体化学の基礎
- 著者
- 中原 勝儼
- 出版者
- 一般社団法人 表面技術協会
- 雑誌
- 金属表面技術 (ISSN:00260614)
- 巻号頁・発行日
- vol.29, no.3, pp.150-157, 1978-03-01 (Released:2009-10-30)
- 参考文献数
- 9
2 0 0 0 OA 金属材料の高温酸化とその対策
- 著者
- 椙山 正孝
- 出版者
- 一般社団法人 表面技術協会
- 雑誌
- 金属表面技術 (ISSN:00260614)
- 巻号頁・発行日
- vol.10, no.9, pp.323-328, 1959-09-20 (Released:2009-10-30)
- 参考文献数
- 19
- 被引用文献数
- 1
2 0 0 0 OA 塗装前処理の最近の進歩
- 著者
- 永井 由太郎
- 出版者
- 一般社団法人 表面技術協会
- 雑誌
- 金属表面技術 (ISSN:00260614)
- 巻号頁・発行日
- vol.11, no.10, pp.478-480, 1960-10-20 (Released:2009-10-30)
- 参考文献数
- 6
2 0 0 0 OA リン酸と金属の反応について
- 著者
- 佐治 孝
- 出版者
- 一般社団法人 表面技術協会
- 雑誌
- 金属表面技術 (ISSN:00260614)
- 巻号頁・発行日
- vol.28, no.1, pp.2-11, 1977-01-01 (Released:2009-10-30)
- 参考文献数
- 76
- 被引用文献数
- 1 2
2 0 0 0 OA メスバウアー分光法と表面分析
- 著者
- 片田 元己 佐野 博敏
- 出版者
- 一般社団法人 表面技術協会
- 雑誌
- 金属表面技術 (ISSN:00260614)
- 巻号頁・発行日
- vol.37, no.3, pp.94-102, 1986-03-01 (Released:2009-10-30)
- 参考文献数
- 12
2 0 0 0 エチレンジアミン浴よりのコバルトメッキ
- 著者
- 津留 壽昭 木村 哲二 乾 忠孝
- 出版者
- The Surface Finishing Society of Japan
- 雑誌
- 金属表面技術 (ISSN:00260614)
- 巻号頁・発行日
- vol.27, no.3, pp.130-134, 1976
A study was made on electrodeposition of cobalt from cobalt ethylenediamine chelate solution for the purpose of investigating the stability of chelate on pH and the optimum condition in electrolysis. The results obtained were as follows: 1) Ethylenediamine (en) and cobalt ion (II) formed stable chelate compound of (Coen<sub>3</sub>)<sup>2+</sup> in a pH range 8.0-10. 2) Bright cobalt platings were obtained from chelate solutions of pH 3.0-5.0 by the addition of two addition agents, and cathodic current efficiency was found to be decreased. The optimum bath composition for bright cobalt plating was as follows: CoCl<sub>2</sub>⋅6H<sub>2</sub>O (CoSO<sub>4</sub>⋅7H<sub>2</sub>O) 0.05-0.20mol/<i>l</i> (0.1-0.3mol/<i>l</i>), en 0.15-0.60mol/<i>l</i> (0.3-0.9mol/<i>l</i>), HOCH<sub>2</sub>C: CCH<sub>2</sub>OH 0.05g/<i>l</i>, C<sub>10</sub>H<sub>6</sub> (SO<sub>3</sub>Na)<sub>2</sub>⋅2H<sub>2</sub>O 0.03g/<i>l</i>, pH 3.0-5.0, Temperature Room temperature, Cathodic current density 0.8-1.5A/dm<sup>2</sup>. The cathodic current efficiency under the above condition was about 70-80%. 3) The cobalt deposits obtained from Co-en baths were of small grains in crystal structure, and the brightness apparently increased with increasing electrolysis time.
2 0 0 0 エチレンジアミン浴よりのカドミウムメッキ
- 著者
- 津留 壽昭 木村 哲二 乾 忠孝
- 出版者
- The Surface Finishing Society of Japan
- 雑誌
- 金属表面技術 (ISSN:00260614)
- 巻号頁・発行日
- vol.27, no.2, pp.80-84, 1976
The stability of electrolyte containing cadmium-ethylenediamine (en) chelate in various pH ranges has been investigated to determine the optimum condition for electrodeposition of cadmium. Cathodic polarization measurement was made in the above bath, and the cadmium deposits obtained under various conditions were examined by X-ray diffraction. The results obtained were as follows: 1) Cadmium (II) ions seemed to react with en to form a stable complex, and bright and smooth cadmium deposits on copper plates were obtained over the range of pH 9-12. 2) Complex ions were greatly affected by anion. Cadmium was not deposited from acidic baths containing a small amount of chloride ions and various precipitates such as Cd(en)<sub>m</sub>Cl<sub>2</sub>(<i>m</i>=1-3), but was deposited from neutral and weak acidic baths. Cadmium-en complex was not formed, when pH-value was in acidic or strong alkaline ranges. In this case, en seemed to act merely as an addition agent upon electrolysis. 3) By the addition of gelatine in to the cadmium-en bath, adherent cadmium deposits having a fine grain (270-290Å) structure were obtained. It was shown by X-ray diffraction analysis that the orientation of the cadmium deposits was strong in (101), but weak in (102) and (103). 4) The optimum composition and operation condition of the bath were found to be Cd (CH<sub>3</sub>COO)<sub>2</sub>⋅2H<sub>2</sub>O: 0.3-0.5mol/<i>l</i>, en: 1.2-2.0mol/<i>l</i>, gelatine: 0.5-1.0g/<i>l</i>, pH: 9-11, temperature: 20-50°C, current density: 1.0-2.5A/dm<sup>2</sup>; current efficiency was above 90%.
2 0 0 0 OA 南米の視察より歸りて
- 著者
- 岡安 彦三郎
- 出版者
- 一般社団法人 表面技術協会
- 雑誌
- 金属表面技術 (ISSN:00260614)
- 巻号頁・発行日
- vol.3, no.3, pp.87-91, 1952-06-20 (Released:2009-10-30)
1 0 0 0 OA ショットピーニング
- 著者
- 加藤 博雄
- 出版者
- 一般社団法人 表面技術協会
- 雑誌
- 金属表面技術 (ISSN:00260614)
- 巻号頁・発行日
- vol.16, no.3, pp.116-122, 1965-03-20 (Released:2009-10-30)
- 参考文献数
- 107
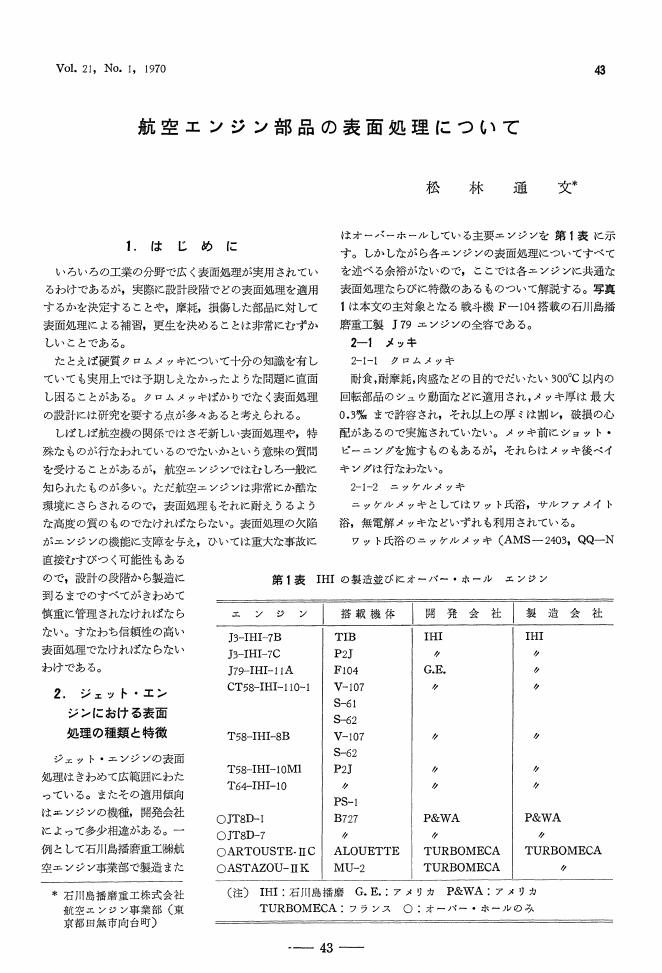
1 0 0 0 OA 航空エンジン部品の表面処理について
- 著者
- 松林 通文
- 出版者
- 一般社団法人 表面技術協会
- 雑誌
- 金属表面技術 (ISSN:00260614)
- 巻号頁・発行日
- vol.21, no.1, pp.43-48, 1970-01-20 (Released:2009-10-30)
1 0 0 0 OA 亜鉛板による写真製版食刻について
- 著者
- 水野 文夫
- 出版者
- 一般社団法人 表面技術協会
- 雑誌
- 金属表面技術 (ISSN:00260614)
- 巻号頁・発行日
- vol.17, no.1, pp.27-34, 1966-01-20 (Released:2009-10-30)
- 参考文献数
- 12
- 著者
- 柏原 太郎 加藤 敏春 有馬 純治 毛利 信幸
- 出版者
- 一般社団法人 表面技術協会
- 雑誌
- 金属表面技術 (ISSN:00260614)
- 巻号頁・発行日
- vol.24, no.9, pp.500-505, 1973-09-01 (Released:2009-10-30)
- 参考文献数
- 7
- 被引用文献数
- 1 1
The mechanism for formation of Cr6+ compounds which were produced by high temperature oxidation reaction of Cr-Ca and Cr(OH)3 systems was investigated. The following conclusions were drawn: (1) When Cr(OH)3-Ca(OH)2 system was burned, Cr6+ compounds were produced by two different mechanisms, which were distinguished by the boundary temperature of 300°C. At temperatures above 300°C, Cr6+ compounds were produced by the mechanism proposed by Nishino et al.; but at temperatures below 300°C, Cr6+ compounds were produced by the following reaction: 4Cr(OH)3+4Ca(OH)3+3O2→4CaCrO4+10H2O (2) CrO3 was prouced by burning of Cr(OH)3 including oxidable anions such as SO42+; and the amount of CrO3 compounds produced was maximum at 250°C. Therefore, it was impossible to prevent the production of Cr6+ compounds in the low temperature burning of real sludge. (3) When Cr-Ca system was burned, the amount of Cr6+ compounds produced became constant at a time at a temperature of 440-600°C; but, at temperatures above 700°C, the surface began to fuse and the reaction proceeded to the interior of particles so that the amount of Cr6+ compounds produced increased with the rise of temperature.
1 0 0 0 OA 銀の変色, 腐食に対するベンゾトリアゾールの抑制効果
- 著者
- 土肥 信康 加藤 敏春 正木 征史
- 出版者
- 一般社団法人 表面技術協会
- 雑誌
- 金属表面技術 (ISSN:00260614)
- 巻号頁・発行日
- vol.26, no.9, pp.411-415, 1975-09-01 (Released:2009-10-30)
- 参考文献数
- 10
- 被引用文献数
- 8 7
The inhibition effects and mechanism of benzotriazole (B.T.A.) on tarnishing and corrosion of silver were studied by means of potentiostatic polarization curves, differential capacity-electrode potential curves, and accelerated testings in H2S and SO2 atmospheres. It was recognized that B.T.A. had little inhibition effects in acidic solutions (pH 2.5), but had great effects in almost neutral solutions; because, B.T.A.-Ag complex was formed on the surface in anodic potential region, and B.T.A. was adsorbed on the surface with lone electron-pairs of N-atoms in cathodic potential region. Silver treated with B.T.A. had an enhanced resistance to tarnishing in H2S atmosphere. It was considered that the prevention would be due to the formation of stable B.T.A.-Ag film on the surface. The galvanic corrosion of silver-plated panels occurred in SO2 atmosphere, leading to the copper base owing to the presence of pores, was much more markedly inhibited by electrolytic treatment than immersion treatment in B.T.A. solution. It was found that the change in the surface coverage (θ) of B.T.A. treated copper conformed to Langmuir's adsorption formula expressed as follows: θ=Km/(1+Km), where K: adsorption constant and m: concentration of B.T.A. Then, the changes of K by immersion treatment and by electrolytic treatment were measured. As the results, a larger value of K was obtained by electrolytic treatment. Therefore, it was considered that the adsorption of B.T.A. on copper through pores became easier by electrolytic treatment.
1 0 0 0 OA 開放循環冷却水系におけるスケール防止技術
- 著者
- 山本 大輔 植木 泱 高橋 知行
- 出版者
- 一般社団法人 表面技術協会
- 雑誌
- 金属表面技術 (ISSN:00260614)
- 巻号頁・発行日
- vol.29, no.5, pp.231-236, 1978-05-01 (Released:2009-10-30)
- 参考文献数
- 8
- 被引用文献数
- 1
1 0 0 0 OA 接点材料の変遷
- 著者
- R. G. Baker T. A. Palumbo 山本 壮兵衛
- 出版者
- 一般社団法人 表面技術協会
- 雑誌
- 金属表面技術 (ISSN:00260614)
- 巻号頁・発行日
- vol.34, no.6, pp.249-253, 1983-06-01 (Released:2009-10-30)
- 参考文献数
- 40
- 被引用文献数
- 1 1