- 著者
- Yukio Suga Mayako Uchida Shinya Suzuki Hideki Sugawara Kazuhiro Torigoe Akihiko Futamura Yoshihiro Uesawa Takayuki Nakagawa Hisamitsu Takase
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.42, no.5, pp.801-806, 2019-05-01 (Released:2019-05-01)
- 参考文献数
- 24
- 被引用文献数
- 10
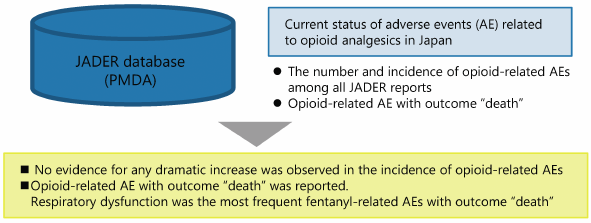
Opioid analgesics have greatly contributed to the advancement of pain management. However, although opioids have been appropriately used in Japan, they rarely induce serious adverse events, such as respiratory depression. The present study aimed to investigate the temporal changes in the occurrence of opioid-related adverse events and deaths between 2004 and 2017 in Japan using the Japanese Adverse Drug Event Report (JADER) database. We analyzed the following points using data extracted from JADER website: 1) temporal changes in the number and proportion of opioid-related adverse event reports; 2) temporal changes in the number of morphine-, oxycodone-, and fentanyl-related adverse event reports per annual consumption; and 3) cases in which the reported outcome following opioid-related adverse events was death. Our results showed no dramatic changes in the overall incidence of opioid-related adverse events, despite the temporal changes in the annual consumption and shared component of each opioid during the survey period. However, the number and rate of fentanyl-related adverse events and their outcome “death” increased since 2010, being the highest among all adverse event including those related to morphine and oxycodone. Outcome “death” by fentanyl-related adverse events was caused mainly due to respiratory depression. These findings suggest that, although opioid-related adverse events can be controlled through proper monitoring and management by medical personnel in Japan, extra caution should be continuously paid for the rare but serious fentanyl-induced adverse events.
- 著者
- Akihiko Futamura Takashi Higashiguchi Takeshi Chihara Yuka Yokota Yoshinori Itani
- 出版者
- Fujita Medical Society
- 雑誌
- Fujita Medical Journal (ISSN:21897247)
- 巻号頁・発行日
- pp.2020-005, (Released:2020-10-10)
- 参考文献数
- 25
Objectives: We have observed white turbidity when a midazolam injection is administered from a lateral tube during the administration of a peripheral parenteral nutrition (PPN) solution. The aim of the current study was to determine how to avoid compound changes when co-administering a midazolam injection and a PPN solution.Methods: Midazolam solutions were prepared by diluting a midazolam injection with a 5% glucose intravenous infusion. We examined the formulation of the midazolam injection and a PPN solution at the concentrations used in a clinical setting for changes in appearance, pH, and midazolam content in test tubes and during administration conditions.Results: With a 1/4.8 dilution of midazolam in undiluted solution, clouding occurred. A strong correlation was revealed between the midazolam content as measured through high-performance liquid chromatography and the mixture’s midazolam concentration (R2=0.9918). The capture rate of midazolam infused with PPN solution was 91.0% at a 1/6 dilution, whereas it decreased to <90% at a 1/4.8 dilution.Conclusions: Our results suggest that the administration of a midazolam injection solution diluted by ≥6-fold with glucose solution or saline from a side tube during the administration of a PPN solution did not cause changes in composition.
- 著者
- Takahiro Hayashi Saori Ikehata Haruna Matsuzaki Kimio Yasuda Toshiyasu Makihara Akihiko Futamura Yuki Arakawa Rika Kuki Kumiko Fukuura Hiroshi Takahashi Naoharu Mori Takashi Higashiguchi Shigeki Yamada
- 出版者
- 公益社団法人日本薬学会
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.37, no.12, pp.1860-1865, 2014-12-01 (Released:2014-12-01)
- 参考文献数
- 25
- 被引用文献数
- 3 7
Morphine, oxycodone, and fentanyl are commonly used to control cancer pain. Because these drugs have differences in receptor affinity or pharmacokinetic parameters, changing the opioid formulation may result in an unexpected outcome, depending on the patient’s condition. This study investigated whether low serum protein levels influence the effectiveness of opioid rotation by determining the impact of serum albumin levels on the analgesic effect before and after opioid rotation from morphine or oxycodone to fentanyl in cancer patients. The patients were classified into 3 groups according to their serum albumin levels before opioid rotation: group 1, <2.5 g/dL; group 2, from 2.5 g/dL to <3.0 g/dL; and group 3, ≥3.0 g/dL. There was no significant change in the percentage of patients with good pain control after rotation in group 1 or group 2; however, the percentage of patients with good pain control increased significantly in group 3. When the percentage of patients whose numerical rating scale scores increased, were unchanged, or decreased after rotation were compared, a significant difference in the percentage of those showing improvement was noted among the 3 groups and between groups 1 and 3. These findings suggest that monitoring serum albumin levels during fentanyl therapy is useful for pain management, and that the effectiveness of opioid rotation to fentanyl in patients with serum albumin levels of <2.5 g/dL should be carefully evaluated after rotation.