- 著者
- Mika Sakamoto Hirofumi Suzuki Kei Yura
- 出版者
- The Biophysical Society of Japan
- 雑誌
- Biophysics and Physicobiology (ISSN:21894779)
- 巻号頁・発行日
- vol.16, pp.68-79, 2019 (Released:2019-02-15)
- 参考文献数
- 41
- 被引用文献数
- 3 4
Transport of small molecules across the cell membrane is a crucial biological mechanism for the maintenance of the cell activity. ABC transporter family is a huge group in the transporter membrane proteins and actively transports the substrates using the energy derived from ATP hydrolysis. In humans, there are 48 distinct genes for ABC transporters. A variation of a single amino acid in the amino acid sequence of ABC transporter has been known to be linked with certain disease. The mechanism of the onset of the disease by the variation is, however, still unclear. Recent progress in the method to measure the structures of huge membrane proteins has enabled determination of the 3D structures of ABC transporters and the accumulation of coordinate data of ABC transporter has enabled us to obtain clues for the onset of the disease caused by a single variation of amino acid residue. We compared the structures of ABC transporter in apo and ATP-binding forms and found a possible conformation shift around pivot-like residues in the transmembrane domains. When this conformation change in ABC transporter and the location of pathogenic variation were compared, we found a reasonable match between the two, explaining the onset of the disease by the variation. They likely cause impairment of the pivot-like movement, weakening of ATP binding and weakening of membrane surface interactions. These findings will give a new interpretation of the variations on ABC transporter genes and pave a way to analyse the effect of variation on protein structure and function.
- 著者
- Ha T. T. Duong Hirofumi Suzuki Saki Katagiri Mayu Shibata Misae Arai Kei Yura
- 出版者
- The Biophysical Society of Japan
- 雑誌
- Biophysics and Physicobiology (ISSN:21894779)
- 巻号頁・発行日
- vol.19, pp.e190025, 2022 (Released:2022-08-20)
- 参考文献数
- 46
Sequencing of individual human genomes enables studying relationship among nucleotide variations, amino acid substitutions, effect on protein structures and diseases. Many studies have found general tendencies, for instance, that pathogenic variations tend to be found in the buried regions of the protein structures, that benign variations tend to be found on the surface of the proteins, and that variations on evolutionary conserved residues tend to be pathogenic. These tendencies were deduced from globular proteins with standard evolutionary changes in amino acid sequences. In this study, we investigated the variation distribution on actin, one of the highly conserved proteins. Many nucleotide variations and three-dimensional structures of actin have been registered in databases. By combining those data, we found that variations buried inside the protein were rather benign and variations on the surface of the protein were pathogenic. This idiosyncratic distribution of the variation impact is likely ascribed to the extensive use of the surface of the protein for protein-protein interactions in actin.
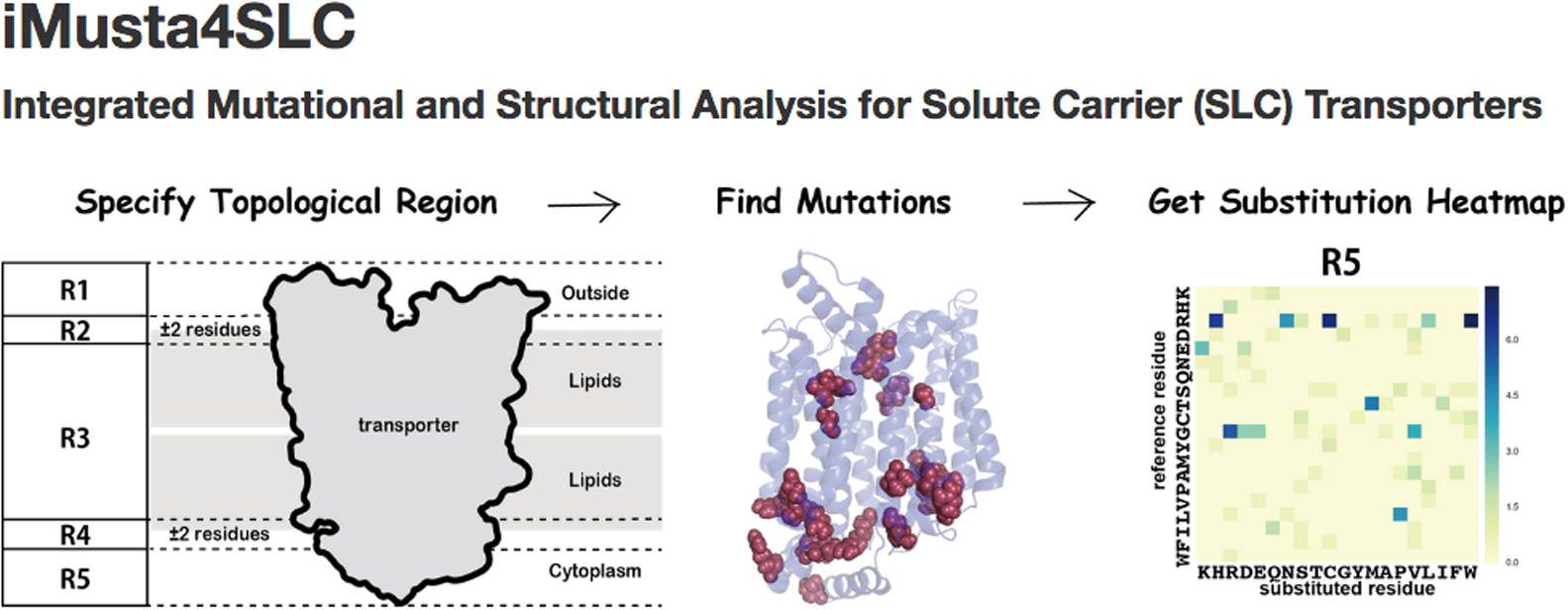
1 0 0 0 OA iMusta4SLC: Database for the structural property and variations of solute carrier transporters
- 著者
- Akiko Higuchi Naoki Nonaka Kei Yura
- 出版者
- The Biophysical Society of Japan
- 雑誌
- Biophysics and Physicobiology (ISSN:21894779)
- 巻号頁・発行日
- vol.15, pp.94-103, 2018 (Released:2018-04-27)
- 参考文献数
- 50
- 被引用文献数
- 7
Membrane transporter proteins play important roles in transport of nutrients into the cell, in transport of waste out of the cell, in maintenance of homeostasis, and in signal transduction. Solute carrier (SLC) transporter is the superfamily, which has the largest number of genes (>400 in humans) in membrane transporter and consists of 52 families. SLC transporters carry a wide variety of substrates such as amino acids, peptides, saccharides, ions, neurotransmitters, lipids, hormones and related materials. Despite the apparent importance for the substrate transport, the information of sequence variation and three-dimensional structures have not been integrated to the level of providing new knowledge on the relationship to, for instance, diseases. We, therefore, built a new database named iMusta4SLC, which is available at http://cib.cf.ocha.ac.jp/slc/, that connected the data of structural properties and of pathogenic mutations on human SLC transporters. iMusta4SLC helps to investigate the structural features of pathogenic mutations on SLC transporters. With this database, we found that the mutations at the conserved arginine were frequently involved in diseases, and were located at a border between the membrane and the cytoplasm. Especially in SLC families 2 and 22, the conserved residues formed a large cluster at the border. In SLC2A1, one third of the reported pathogenic missense mutations were found in this conserved cluster.
- 著者
- Kei Yura
- 出版者
- 一般社団法人 日本生物物理学会
- 雑誌
- Biophysics and Physicobiology (ISSN:21894779)
- 巻号頁・発行日
- vol.13, pp.245-247, 2016 (Released:2016-11-18)
- 参考文献数
- 13
- 被引用文献数
- 1 2
- 著者
- Yuka Suzuki Kei Yura
- 出版者
- 一般社団法人 日本生物物理学会
- 雑誌
- Biophysics and Physicobiology (ISSN:21894779)
- 巻号頁・発行日
- vol.13, pp.127-134, 2016 (Released:2016-07-14)
- 参考文献数
- 26
- 被引用文献数
- 4
We investigated the effect of ATP binding to GroEL and elucidated a role of ATP in the conformational change of GroEL. GroEL is a tetradecamer chaperonin that helps protein folding by undergoing a conformational change from a closed state to an open state. This conformational change requires ATP, but does not require the hydrolysis of the ATP. The following three types of conformations are crystalized and the atomic coordinates are available; closed state without ATP, closed state with ATP and open state with ADP. We conducted simulations of the conformational change using Elastic Network Model from the closed state without ATP targeting at the open state, and from the closed state with ATP targeting at the open state. The simulations emphasizing the lowest normal mode showed that the one started with the closed state with ATP, rather than the one without ATP, reached a conformation closer to the open state. This difference was mainly caused by the changes in the positions of residues in the initial structure rather than the changes in “connectivity” of residues within the subunit. Our results suggest that ATP should behave as an insulator to induce conformation population shift in the closed state to the conformation that has a pathway leading to the open state.
- 著者
- Saki Aoto Kei Yura
- 出版者
- 一般社団法人 日本生物物理学会
- 雑誌
- Biophysics and Physicobiology (ISSN:21894779)
- 巻号頁・発行日
- vol.12, pp.103-116, 2015 (Released:2015-12-02)
- 参考文献数
- 69
- 被引用文献数
- 2
We addressed the evolutionary trace of hetero-oligomer interfaces by comparing the structures of paralogous proteins; one of them is a monomer or homo-oligomer and the other is a hetero-oligomer. We found different trends in amino acid conservation pattern and hydrophobicity between homo-oligomer and hetero-oligomer. The degree of amino acid conservation in the interface of homo-oligomer has no obvious difference from that in the surface, whereas the degree of conservation is much higher in the interface of hetero-oligomer. The interface of homo-oligomer has a few very conserved residue positions, whereas the residue conservation in the interface of hetero-oligomer tends to be higher. In addition, the interface of hetero-oligomer has a tendency of being more hydrophobic compared with the one in homo-oligomer. We conjecture that these differences are related to the inherent symmetry in homo-oligomers that cannot exist in hetero-oligomers. Paucity of the structural data precludes statistical tests of these tendencies, yet the trend can be applied to the prediction of the interface of hetero-oligomer. We obtained putative interfaces of the subunits in CPSF (cleavage and polyadenylation specificity factor), one of the human pre-mRNA 3’-processing complexes. The locations of predicted interface residues were consistent with the known experimental data.