- 著者
- Tohru Minamino Miki Kinoshita Yusuke V. Morimoto Keiichi Namba
- 出版者
- The Biophysical Society of Japan
- 雑誌
- Biophysics and Physicobiology (ISSN:21894779)
- 巻号頁・発行日
- pp.e190046, (Released:2022-11-19)
- 被引用文献数
- 3
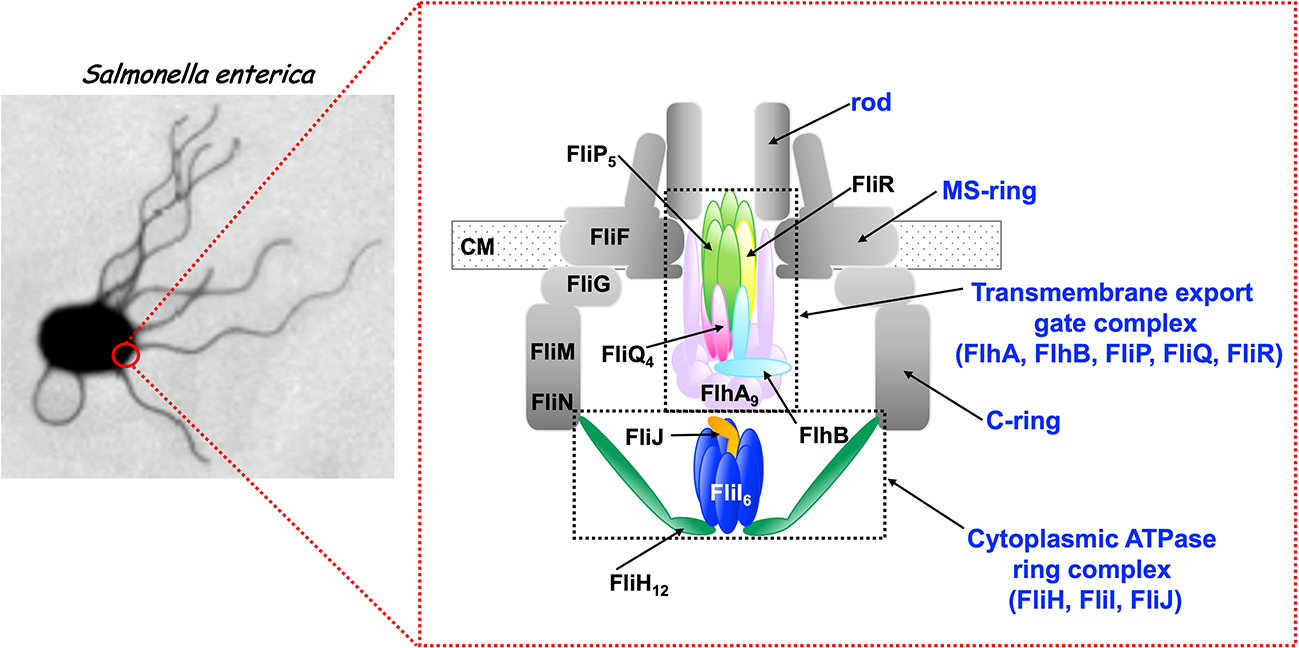
Bacteria employ the flagellar type III secretion system (fT3SS) to construct flagellum, which acts as a supramolecular motility machine. The fT3SS of Salmonella enterica serovar Typhimurium is composed of a transmembrane export gate complex and a cytoplasmic ATPase ring complex. The transmembrane export gate complex is fueled by proton motive force across the cytoplasmic membrane and is divided into four distinct functional parts: a dual-fuel export engine; a polypeptide channel; a membrane voltage sensor; and a docking platform. ATP hydrolysis by the cytoplasmic ATPase complex converts the export gate complex into a highly efficient proton (H+)/ protein antiporter that couples inward-directed H+ flow with outward-directed protein export. When the ATPase ring complex does not work well in a given environment, the export gate complex will remain inactive. However, when the electric potential difference, which is defined as membrane voltage, rises above a certain threshold value, the export gate complex becomes an active H+/protein antiporter to a considerable degree, suggesting that the export gate complex has a voltage-gated activation mechanism. Furthermore, the export gate complex also has a sodium ion (Na+) channel to couple Na+ influx with flagellar protein export. In this article, we review our current understanding of the activation mechanism of the dual-fuel protein export engine of the fT3SS. This review article is an extended version of a Japanese article, Membrane voltage-dependent activation of the transmembrane export gate complex in the bacterial flagellar type III secretion system, published in SEIBUTSU BUTSURI Vol. 62, p165–169 (2022).
- 著者
- Tohru Minamino Miki Kinoshita Yusuke V. Morimoto Keiichi Namba
- 出版者
- The Biophysical Society of Japan
- 雑誌
- Biophysics and Physicobiology (ISSN:21894779)
- 巻号頁・発行日
- vol.19, pp.e190046, 2022 (Released:2022-12-07)
- 参考文献数
- 83
- 被引用文献数
- 3
Bacteria employ the flagellar type III secretion system (fT3SS) to construct flagellum, which acts as a supramolecular motility machine. The fT3SS of Salmonella enterica serovar Typhimurium is composed of a transmembrane export gate complex and a cytoplasmic ATPase ring complex. The transmembrane export gate complex is fueled by proton motive force across the cytoplasmic membrane and is divided into four distinct functional parts: a dual-fuel export engine; a polypeptide channel; a membrane voltage sensor; and a docking platform. ATP hydrolysis by the cytoplasmic ATPase complex converts the export gate complex into a highly efficient proton (H+)/protein antiporter that couples inward-directed H+ flow with outward-directed protein export. When the ATPase ring complex does not work well in a given environment, the export gate complex will remain inactive. However, when the electric potential difference, which is defined as membrane voltage, rises above a certain threshold value, the export gate complex becomes an active H+/protein antiporter to a considerable degree, suggesting that the export gate complex has a voltage-gated activation mechanism. Furthermore, the export gate complex also has a sodium ion (Na+) channel to couple Na+ influx with flagellar protein export. In this article, we review our current understanding of the activation mechanism of the dual-fuel protein export engine of the fT3SS. This review article is an extended version of a Japanese article, Membrane voltage-dependent activation of the transmembrane export gate complex in the bacterial flagellar type III secretion system, published in SEIBUTSU BUTSURI Vol. 62, p165–169 (2022).