- 著者
- Toshihiro TAKAMI Takeshi HARA Masahito HARA Toshihiko INUI Kiyoshi ITO Izumi KOYANAGI Junichi MIZUNO Masaki MIZUNO Hiroyuki NAKASE Nobuyuki SHIMOKAWA Taku SUGAWARA Shinsuke SUZUKI Toshiyuki TAKAHASHI Masakazu TAKAYASU Satoshi TANI Kazutoshi HIDA Phyo KIM Hajime ARAI Neurospinal Society of Japan The Japan Neurosurgical Society
- 出版者
- The Japan Neurosurgical Society
- 雑誌
- Neurologia medico-chirurgica (ISSN:04708105)
- 巻号頁・発行日
- pp.2022-0148, (Released:2022-10-13)
- 参考文献数
- 74
- 被引用文献数
- 2
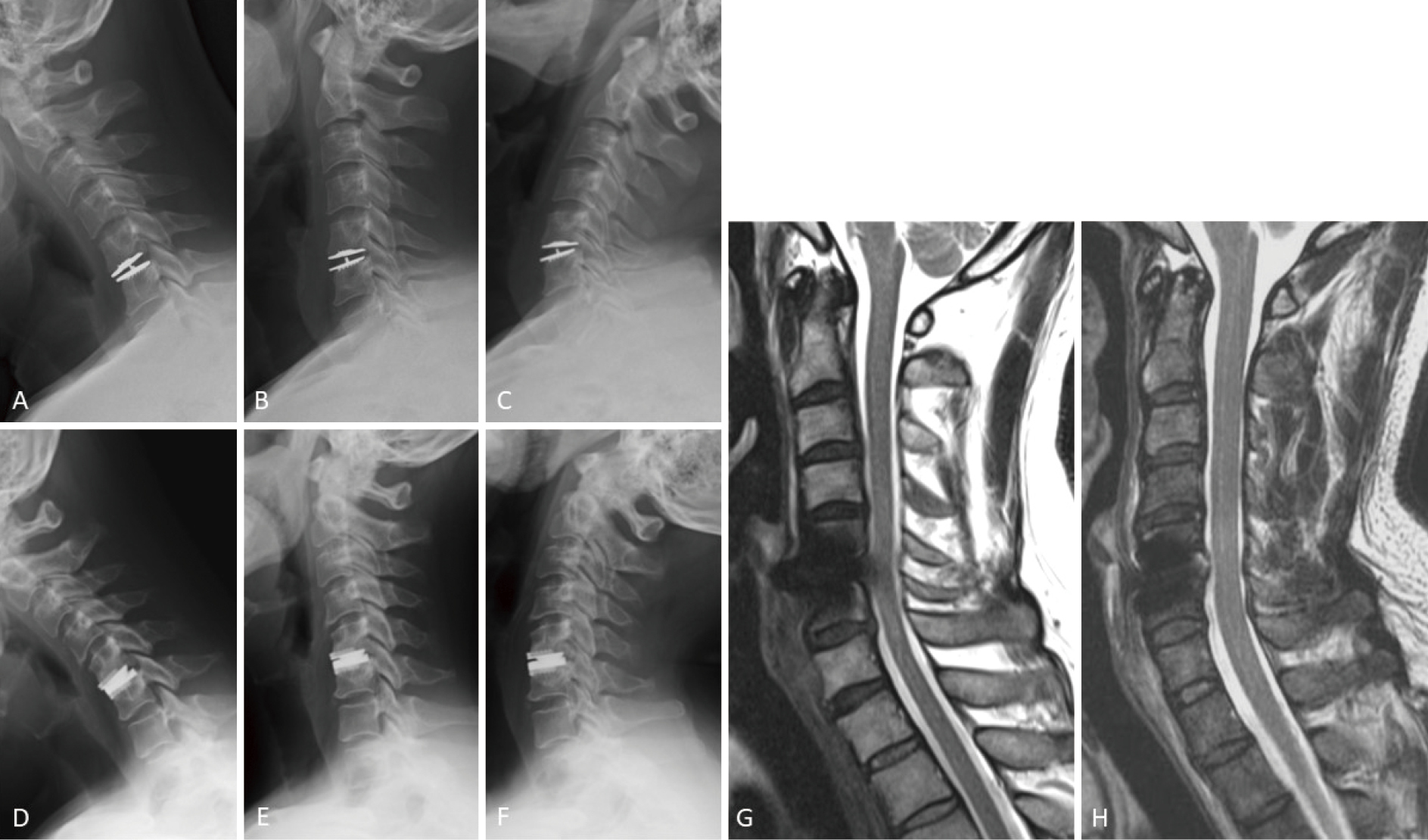
Anterior cervical disc replacement (ACDR) using cervical artificial disc (CAD) has the advantage of maintaining the range of motion (ROM) at the surgical level, subsequently reducing the postoperative risk of adjacent disc disease. Following the approval for the clinical use in Japan, a post-marketing surveillance (PMS) study was conducted for two different types of CAD, namely, Mobi-C (metal-on-plastic design) and Prestige LP (metal-on-metal design). The objective of this prospective observational multicenter study was to analyze the first 2-year surgical results of the PMS study of 1-level ACDR in Japan. A total of 54 patients were registered (Mobi-C, n = 24, MC group; Prestige LP, n = 30, PLP group). Preoperative neurological assessment revealed radiculopathy in 31 patients (57.4%) and myelopathy in 15 patients (27.8%). Preoperative radiological assessment classified the disease category as disc herniation in 15 patients (27.8%), osteophyte in 6 patients (11.1%), and both in 33 patients (61.1%). The postoperative follow-up rates at 6 weeks, 6 months, 1 year, and 2 years after ACDR were 92.6%, 87.0%, 83.3%, and 79.6%, respectively. In both groups, patients' neurological condition improved significantly after surgery. Radiographic assessment revealed loss of mobility at the surgical level in 9.5% of patients in the MC group and in 9.1% of patients in the PLP group. No secondary surgeries at the initial surgical level and no serious adverse events were observed in either group. The present results suggest that 1-level ACDR is safe, although medium- to long-term follow-up is mandatory to further verify the validity of ACDR for Japanese patients.