- 著者
- Toshihiro TAKAMI Takeshi HARA Masahito HARA Toshihiko INUI Kiyoshi ITO Izumi KOYANAGI Junichi MIZUNO Masaki MIZUNO Hiroyuki NAKASE Nobuyuki SHIMOKAWA Taku SUGAWARA Shinsuke SUZUKI Toshiyuki TAKAHASHI Masakazu TAKAYASU Satoshi TANI Kazutoshi HIDA Phyo KIM Hajime ARAI Neurospinal Society of Japan The Japan Neurosurgical Society
- 出版者
- The Japan Neurosurgical Society
- 雑誌
- Neurologia medico-chirurgica (ISSN:04708105)
- 巻号頁・発行日
- pp.2022-0148, (Released:2022-10-13)
- 参考文献数
- 74
- 被引用文献数
- 2
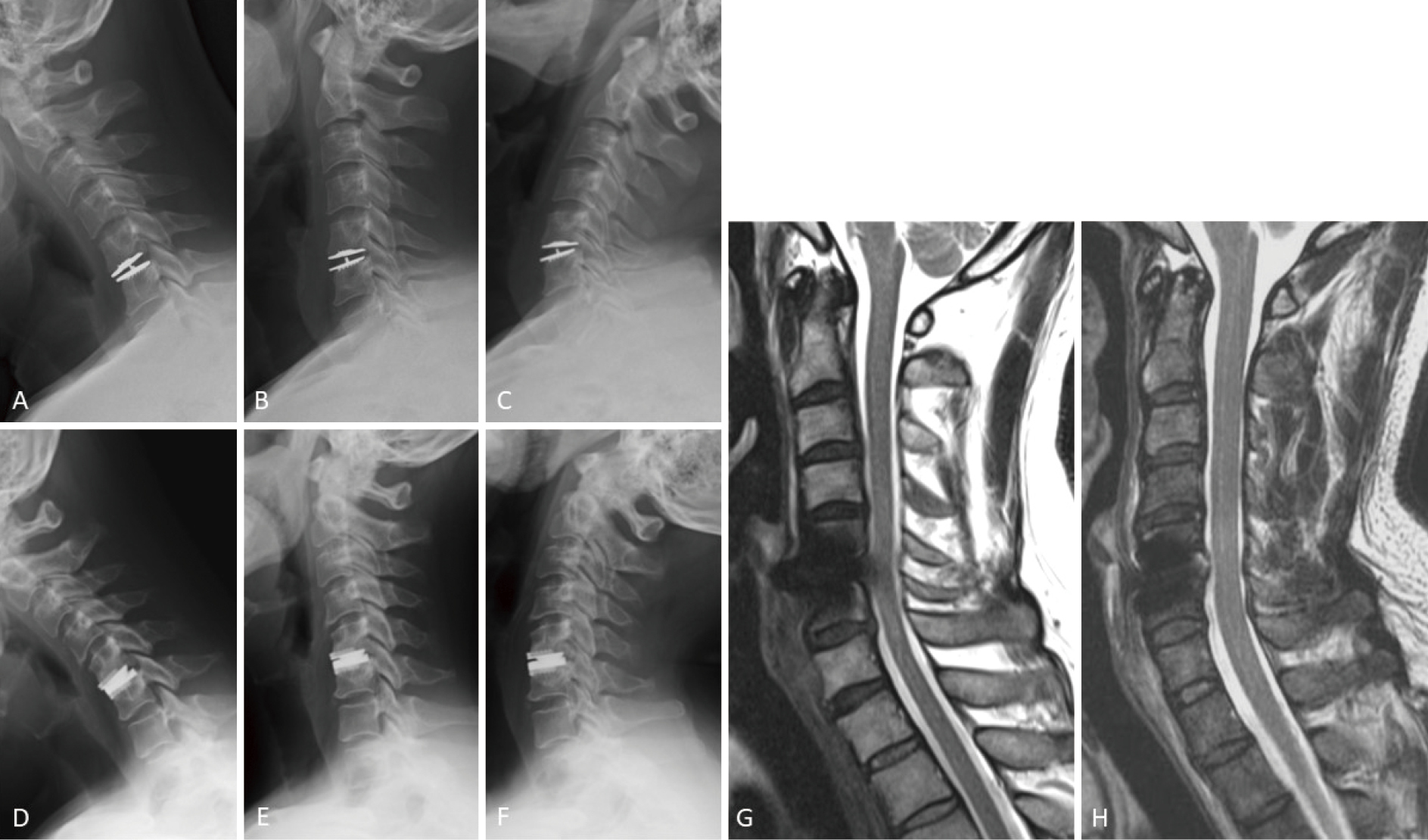
Anterior cervical disc replacement (ACDR) using cervical artificial disc (CAD) has the advantage of maintaining the range of motion (ROM) at the surgical level, subsequently reducing the postoperative risk of adjacent disc disease. Following the approval for the clinical use in Japan, a post-marketing surveillance (PMS) study was conducted for two different types of CAD, namely, Mobi-C (metal-on-plastic design) and Prestige LP (metal-on-metal design). The objective of this prospective observational multicenter study was to analyze the first 2-year surgical results of the PMS study of 1-level ACDR in Japan. A total of 54 patients were registered (Mobi-C, n = 24, MC group; Prestige LP, n = 30, PLP group). Preoperative neurological assessment revealed radiculopathy in 31 patients (57.4%) and myelopathy in 15 patients (27.8%). Preoperative radiological assessment classified the disease category as disc herniation in 15 patients (27.8%), osteophyte in 6 patients (11.1%), and both in 33 patients (61.1%). The postoperative follow-up rates at 6 weeks, 6 months, 1 year, and 2 years after ACDR were 92.6%, 87.0%, 83.3%, and 79.6%, respectively. In both groups, patients' neurological condition improved significantly after surgery. Radiographic assessment revealed loss of mobility at the surgical level in 9.5% of patients in the MC group and in 9.1% of patients in the PLP group. No secondary surgeries at the initial surgical level and no serious adverse events were observed in either group. The present results suggest that 1-level ACDR is safe, although medium- to long-term follow-up is mandatory to further verify the validity of ACDR for Japanese patients.
- 著者
- Yoshitaka NAGASHIMA Yusuke NISHIMURA Tokumi KANEMURA Nobuhiro HATA Kotaro SATAKE Sho AKAHORI Motonori ISHII Takafumi TANEI Masakazu TAKAYASU Ryuta SAITO
- 出版者
- The Japan Neurosurgical Society
- 雑誌
- Neurologia medico-chirurgica (ISSN:04708105)
- 巻号頁・発行日
- pp.2023-0064, (Released:2023-10-18)
- 参考文献数
- 31
There is a lack of agreement on whether minimally invasive lateral lumbar intervertebral fusion (LLIF) is a suitable treatment option for vertebral fragility fractures (VFFs). Hence, we sought to evaluate the efficacy and safety of LLIF in the management of VFF with neurological deficits in the lumbar spine. Between April 2015 and March 2020, we conducted a retrospective observational study of patients with VFF treated with three-level or less LLIF. The participants had previously received conservative treatment but had not been able to control their neurological symptoms. To assess the outcomes of the LLIF procedures, the patients were followed up for a minimum of 1 year. Clinical and radiological results, which include the timing and location of the bony fusion, were analyzed. The study involved 19 patients with 23 vertebral fracture levels. The residual height of the fractured vertebra was found to be 57.0 ± 12.3% of the height of the adjacent level. The mean Japanese Orthopedic Association score significantly improved postoperatively. Postoperative radiological parameters were significantly maintained at 1 year, and lumbar lordosis was maintained at the last follow-up (45.0 ± 26.7). In total 31 LLIF levels, bone fusion was observed in four levels at 6 months postoperatively, in 16 levels at 1 year, and in 23 levels at the last follow-up. The facet joint had the highest bony fusion location. LLIF within three levels can be safely performed in certain VFF cases with sufficient residual vertebral height.
1 0 0 0 OA Surgical Outcomes of Common Peroneal Nerve Entrapment Neuropathy Associated with L5 Radiculopathy
- 著者
- Motonori ISHII Yusuke NISHIMURA Masahito HARA Yu YAMAMOTO Yoshitaka NAGASHIMA Takafumi TANEI Masakazu TAKAYASU Ryuta SAITO
- 出版者
- The Japan Neurosurgical Society
- 雑誌
- Neurologia medico-chirurgica (ISSN:04708105)
- 巻号頁・発行日
- pp.2022-0313, (Released:2023-06-08)
- 参考文献数
- 29
Impingement of the common peroneal nerve, a branch of the L5 nerve root, causes common peroneal nerve entrapment neuropathy (CPNE). Although there are cases of CPNE associated with L5 radiculopathy, surgical intervention's effectiveness remains to be elucidated. This retrospective case-control study aimed to evaluate the efficacy of surgery in patients with CPNE associated with L5 radiculopathy. Twenty-two patients (25 limbs) with surgically treated CPNE between 2015 and 2022 were retrospectively reviewed. The limbs were classified into two groups: group R (limbs of CPNE associated with L5 radiculopathy) and group O (limbs of CPNE without L5 radiculopathy). The durations from onset to surgery, the nerve conduction studies (NCSs), and postoperative improvement rates for motor weakness, pain, and dysesthesia were compared between the groups. Group R included 15 limbs (13 patients), and group O included 10 limbs (9 patients). There were no significant differences in the duration from onset to surgery or abnormal findings of NCS between the two groups. The postoperative improvement rates were 88% and 100% (p = 0.62) for muscle weakness, 87% and 80% (p = 0.53) for pain, and 71% and 56% (p = 0.37) for dysesthesia in group R and group O, respectively, without significant differences between groups. CPNE associated with L5 radiculopathy is common, and the results of the present study showed that the surgical outcomes in such cases were satisfactory and comparable to those in CPNE without L5 radiculopathy.
- 著者
- Satoshi YOSHIKAWA Yusuke NISHIMURA Yoshitaka NAGASHIMA Hiroshi ITO Takahiro OYAMA Tomoya NISHII Tomomi GONDA Hiroshi RYU Kei NOMURA Masahito HARA Masakazu TAKAYASU Howard J GINSBERG Tokumi KANEMURA Ryuta SAITO
- 出版者
- The Japan Neurosurgical Society
- 雑誌
- Neurologia medico-chirurgica (ISSN:04708105)
- 巻号頁・発行日
- pp.2021-0390, (Released:2023-03-01)
- 参考文献数
- 28
The goal of this study is to perform correlation analysis of Computed tomography (CT) and magnetic resonance imaging (MRI) results in posterior ligament complex (PLC) injury and define the morphological traits of thoracolumbar (TL) burst fractures connected to PLC injury. Forty patients with surgically repaired TL burst fractures between January 2013 and December 2020 were retrospectively analyzed. The patients were split into two groups for comparison based on MRI (Group P: patients with a confirmed or suspected PLC injury; Group N: patients with PLC injury denied). The radiographic morphological examination based on CT scans and clinical evaluation was performed and compared between two groups. The thoracolumbar injury classification and severity score (TLICS), the load sharing classification (LSC) scores, and the number of patients with neurological impairments were considerably greater in Group P. Loss of height of the fracture (loss height), local kyphosis of the fracture (local kyphosis), and supraspinous distance were significantly higher in Group P and significantly associated with PLC injuries indicating severe vertebral body destruction and traumatic kyphosis in multivariate logistic analysis [odds ratio: 1.90, 1.06, and 1.13, respectively]. Cutoff value for local kyphosis obtained from the receiver operating characteristic curve was 18.8. If local kyphosis is greater than 18.8 degrees on CT scans, we should take into account the probability of the highly damaged burst fracture associated with PLC injury. In this situation, we should carefully assess MRI to identify the spinal cord injury or spinal cord compression in addition to PLC injury because these instances likely present with neurological abnormalities.