- 著者
- Madoka NAKAJIMA Shigeki YAMADA Masakazu MIYAJIMA Kazunari ISHII Nagato KURIYAMA Hiroaki KAZUI Hideki KANEMOTO Takashi SUEHIRO Kenji YOSHIYAMA Masahiro KAMEDA Yoshinaga KAJIMOTO Mitsuhito MASE Hisayuki MURAI Daisuke KITA Teruo KIMURA Naoyuki SAMEJIMA Takahiko TOKUDA Mitsunobu KAIJIMA Chihiro AKIBA Kaito KAWAMURA Masamichi ATSUCHI Yoshihumi HIRATA Mitsunori MATSUMAE Makoto SASAKI Fumio YAMASHITA Shigeki AOKI Ryusuke IRIE Hiroji MIYAKE Takeo KATO Etsuro MORI Masatsune ISHIKAWA Isao DATE Hajime ARAI The research committee of idiopathic normal pressure hydrocephalus
- 出版者
- The Japan Neurosurgical Society
- 雑誌
- Neurologia medico-chirurgica (ISSN:04708105)
- 巻号頁・発行日
- vol.61, no.2, pp.63-97, 2021 (Released:2021-02-15)
- 参考文献数
- 286
- 被引用文献数
- 88 211
Among the various disorders that manifest with gait disturbance, cognitive impairment, and urinary incontinence in the elderly population, idiopathic normal pressure hydrocephalus (iNPH) is becoming of great importance. The first edition of these guidelines for management of iNPH was published in 2004, and the second edition in 2012, to provide a series of timely, evidence-based recommendations related to iNPH. Since the last edition, clinical awareness of iNPH has risen dramatically, and clinical and basic research efforts on iNPH have increased significantly. This third edition of the guidelines was made to share these ideas with the international community and to promote international research on iNPH. The revision of the guidelines was undertaken by a multidisciplinary expert working group of the Japanese Society of Normal Pressure Hydrocephalus in conjunction with the Japanese Ministry of Health, Labour and Welfare research project. This revision proposes a new classification for NPH. The category of iNPH is clearly distinguished from NPH with congenital/developmental and acquired etiologies. Additionally, the essential role of disproportionately enlarged subarachnoid-space hydrocephalus (DESH) in the imaging diagnosis and decision for further management of iNPH is discussed in this edition. We created an algorithm for diagnosis and decision for shunt management. Diagnosis by biomarkers that distinguish prognosis has been also initiated. Therefore, diagnosis and treatment of iNPH have entered a new phase. We hope that this third edition of the guidelines will help patients, their families, and healthcare professionals involved in treating iNPH.
- 著者
- Etsuro MORI Masatsune ISHIKAWA Takeo KATO Hiroaki KAZUI Hiroji MIYAKE Masakazu MIYAJIMA Madoka NAKAJIMA Masaaki HASHIMOTO Nagato KURIYAMA Takahiko TOKUDA Kazunari ISHII Mitsunobu KAIJIMA Yoshihumi HIRATA Makoto SAITO Hajime ARAI
- 出版者
- The Japan Neurosurgical Society
- 雑誌
- Neurologia medico-chirurgica (ISSN:04708105)
- 巻号頁・発行日
- vol.52, no.11, pp.775-809, 2012 (Released:2012-11-25)
- 参考文献数
- 253
- 被引用文献数
- 149 480
Among the various disorders manifesting dementia, gait disturbance, and urinary incontinence in the elderly population, idiopathic normal pressure hydrocephalus (iNPH) is becoming of great importance. After the publication of the first edition of the Guidelines for Management of Idiopathic Normal Pressure Hydrocephalus in 2004 (the English version was published in 2008), clinical awareness of iNPH has risen dramatically, and the number of shunt surgeries has increased rapidly across Japan. Clinical and basic research on iNPH has increased significantly, and more high-level evidence has since been generated. The second edition of the Japanese Guidelines was thus published in July 2011, to provide a series of timely evidence-based recommendations related to iNPH. The revision of the Guidelines has been undertaken by a multidisciplinary expert working group of the Japanese Society of Normal Pressure Hydrocephalus in conjunction with the Japanese Ministry of Health, Labour and Welfare research project on “Studies on the epidemiology, pathophysiology, and treatment of normal pressure hydrocephalus.” This English version of the second edition of the Guidelines was made to share these ideas with the international community and to promote international research on iNPH.
- 著者
- Toshihiro TAKAMI Takeshi HARA Masahito HARA Toshihiko INUI Kiyoshi ITO Izumi KOYANAGI Junichi MIZUNO Masaki MIZUNO Hiroyuki NAKASE Nobuyuki SHIMOKAWA Taku SUGAWARA Shinsuke SUZUKI Toshiyuki TAKAHASHI Masakazu TAKAYASU Satoshi TANI Kazutoshi HIDA Phyo KIM Hajime ARAI Neurospinal Society of Japan The Japan Neurosurgical Society
- 出版者
- The Japan Neurosurgical Society
- 雑誌
- Neurologia medico-chirurgica (ISSN:04708105)
- 巻号頁・発行日
- pp.2022-0148, (Released:2022-10-13)
- 参考文献数
- 74
- 被引用文献数
- 2
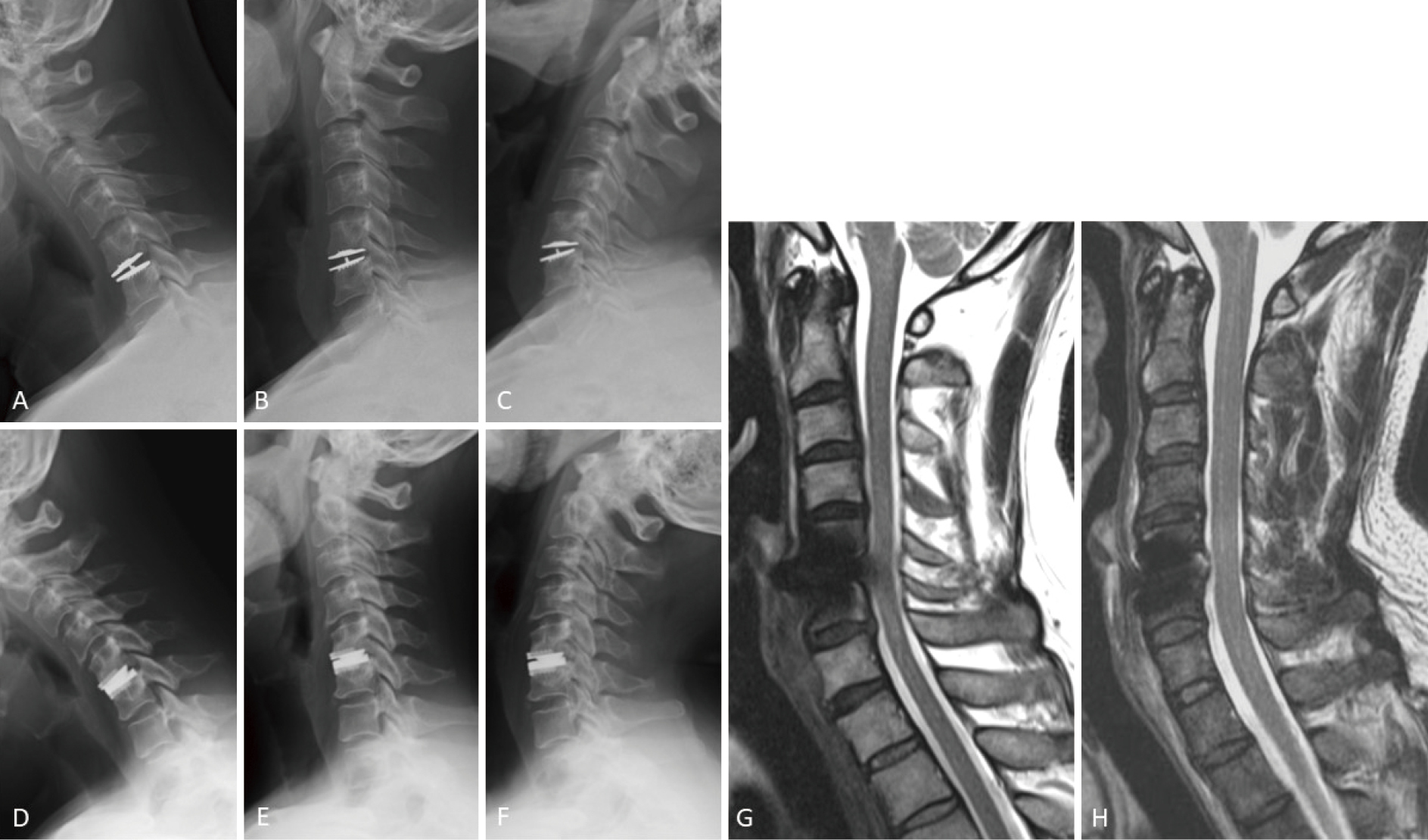
Anterior cervical disc replacement (ACDR) using cervical artificial disc (CAD) has the advantage of maintaining the range of motion (ROM) at the surgical level, subsequently reducing the postoperative risk of adjacent disc disease. Following the approval for the clinical use in Japan, a post-marketing surveillance (PMS) study was conducted for two different types of CAD, namely, Mobi-C (metal-on-plastic design) and Prestige LP (metal-on-metal design). The objective of this prospective observational multicenter study was to analyze the first 2-year surgical results of the PMS study of 1-level ACDR in Japan. A total of 54 patients were registered (Mobi-C, n = 24, MC group; Prestige LP, n = 30, PLP group). Preoperative neurological assessment revealed radiculopathy in 31 patients (57.4%) and myelopathy in 15 patients (27.8%). Preoperative radiological assessment classified the disease category as disc herniation in 15 patients (27.8%), osteophyte in 6 patients (11.1%), and both in 33 patients (61.1%). The postoperative follow-up rates at 6 weeks, 6 months, 1 year, and 2 years after ACDR were 92.6%, 87.0%, 83.3%, and 79.6%, respectively. In both groups, patients' neurological condition improved significantly after surgery. Radiographic assessment revealed loss of mobility at the surgical level in 9.5% of patients in the MC group and in 9.1% of patients in the PLP group. No secondary surgeries at the initial surgical level and no serious adverse events were observed in either group. The present results suggest that 1-level ACDR is safe, although medium- to long-term follow-up is mandatory to further verify the validity of ACDR for Japanese patients.