- 著者
- Akihiko Nakamura Kei-ichi Okazaki Tadaomi Furuta Minoru Sakurai Jun Ando Ryota Iino
- 出版者
- The Biophysical Society of Japan
- 雑誌
- Biophysics and Physicobiology (ISSN:21894779)
- 巻号頁・発行日
- vol.17, pp.51-58, 2020 (Released:2020-07-10)
- 参考文献数
- 26
- 被引用文献数
- 4 5
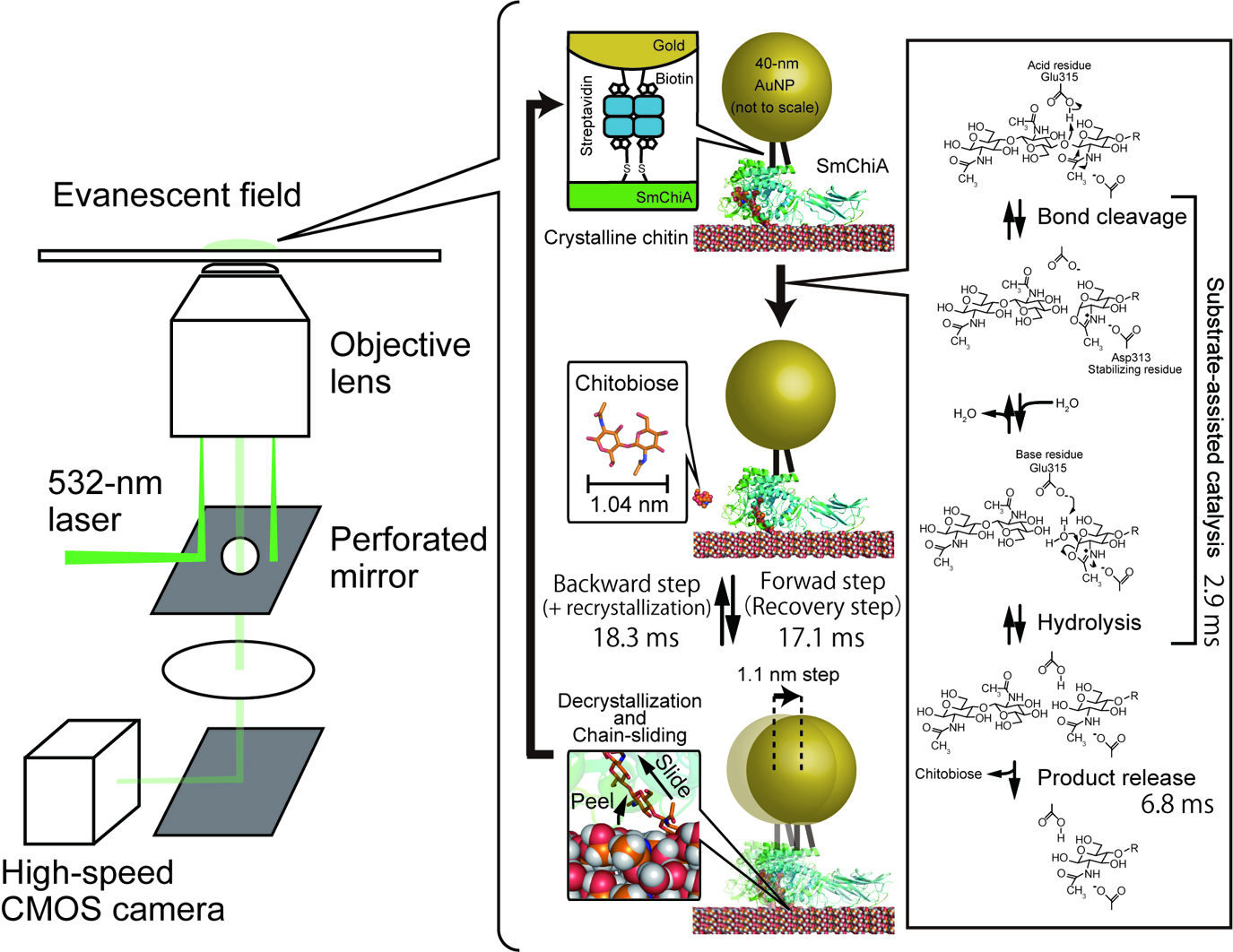
Motor proteins are essential units of life and are well-designed nanomachines working under thermal fluctuations. These proteins control moving direction by consuming chemical energy or by dissipating electrochemical potentials. Chitinase A from bacterium Serratia marcescens (SmChiA) processively moves along crystalline chitin by hydrolysis of a single polymer chain to soluble chitobiose. Recently, we directly observed the stepping motions of SmChiA labeled with a gold nanoparticle by dark-field scattering imaging to investigate the moving mechanism. Time constants analysis revealed that SmChiA moves back and forth along the chain freely, because forward and backward states have a similar free energy level. The similar probabilities of forward-step events (83.5%=69.3%+14.2%) from distributions of step sizes and chain-hydrolysis (86.3%=(1/2.9)/(1/2.9+1/18.3)×100) calculated from the ratios of time constants of hydrolysis and the backward step indicated that SmChiA moves forward as a result of shortening of the chain by a chitobiose unit, which stabilizes the backward state. Furthermore, X-ray crystal structures of sliding intermediate and molecular dynamics simulations showed that SmChiA slides forward and backward under thermal fluctuation without large conformational changes of the protein. Our results demonstrate that SmChiA is a burnt-bridge Brownian ratchet motor.
- 著者
- Akihiro Otomo Lucy Zhu Takanori Harashima Ryota Iino
- 雑誌
- 第61回日本生物物理学会年会
- 巻号頁・発行日
- 2023-10-25
- 著者
- Jakia Jannat Keya Akasit Visootsat Kimitoshi Takeda Akihiro Otomo Wijak Yospanya Sanghun Han Kazushi Kinbara Ryota Iino
- 雑誌
- 第60回日本生物物理学会年会
- 巻号頁・発行日
- 2022-09-02
- 著者
- Akihiko Nakamura Kei-ichi Okazaki Tadaomi Furuta Minoru Sakurai Jun Ando Ryota Iino
- 出版者
- The Biophysical Society of Japan
- 雑誌
- Biophysics and Physicobiology (ISSN:21894779)
- 巻号頁・発行日
- pp.BSJ-2020004, (Released:2020-06-09)
- 被引用文献数
- 5