2 0 0 0 OA 地域の物理的・社会的環境要因が女子大学生の犯罪不安に与える影響
- 著者
- 森 康浩 Yasuhiro Mori
- 雑誌
- 宮城學院女子大學研究論文集 = Bulletin of Miyagi Gakuin Women's University (ISSN:03867501)
- 巻号頁・発行日
- no.128・129, pp.71-84, 2019-12-30
2 0 0 0 OA 佐賀藩大阪蔵屋敷の成立
- 著者
- 森 泰博 Yasuhiro Mori
- 雑誌
- 商学論究 (ISSN:02872552)
- 巻号頁・発行日
- vol.46, no.3, pp.135-146, 1999-03-10
- 著者
- Mayako Uchida Yasuhiro Mori Kenta Akiba Moena Miyasaka Tatsuya Hirano Hiroaki Ikesue Yuki Yamaguchi Aoi Takano Nami Maegawa Yoshimitsu Shimomura Keiko Hosohata Nobuyuki Muroi Takayuki Ishikawa Tohru Hashida Tsutomu Nakamura
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.43, no.10, pp.1577-1582, 2020-10-01 (Released:2020-10-01)
- 参考文献数
- 26
- 被引用文献数
- 2
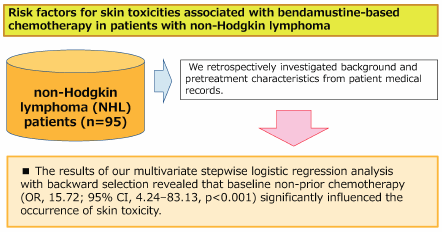
Bendamustine plays an especially important role as a treatment for non-Hodgkin lymphoma (NHL). However, patients administered bendamustine alone or in combination with rituximab (BR) may experience drug-associated skin toxicities that can profoundly impact their health-related QOL through both physical discomfort and psychological distress. Moreover, worsening skin symptoms may lead to dose reduction or termination in the management of cancer chemotherapy. We retrospectively investigated patient backgrounds and pretreatment characteristics from medical records of NHL patients treated with bendamustine alone or BR therapy and identified predictive factors for skin toxicities at the start of chemotherapy. Patients were eligible for the study if they were 20 years older, diagnosed with NHL, and received bendamustine alone or BR therapy at the Department of Hematology, Kobe City Medical Center General Hospital, between April 1, 2011, and March 31, 2018. This study included 95 patients with newly diagnosed or refractory or relapsed NHL. Multivariate stepwise logistic regression analysis with backward selection revealed that baseline non-prior chemotherapy (odds ratio (OR), 15.72; 95% confidence interval (CI), 4.24–83.13, p < 0.001) was a significant factor influencing the occurrence of skin toxicity. Our results demonstrated that non-prior chemotherapy was a significant risk factor for skin toxicities in patients with NHL receiving bendamustine alone or BR therapy. No patient experience serious side effects of grade 3 or higher and that bendamustine is very useful as a first-line treatment.
- 著者
- Mayako Uchida Yuki Yamaguchi Syuhei Hosomi Hiroaki Ikesue Yasuhiro Mori Nami Maegawa Aoi Takano Yuki Sato Keiko Hosohata Nobuyuki Muroi Keisuke Tomii Tohru Hashida Tsutomu Nakamura
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.43, no.8, pp.1235-1240, 2020-08-01 (Released:2020-08-01)
- 参考文献数
- 42
- 被引用文献数
- 3
We retrospectively obtained data of patient background and pretreatment characteristics from medical records and identified the predictive factors of febrile neutropenia (FN) in patients with non-small cell lung cancer (NSCLC) treated with docetaxel alone or in combination with the anti-vascular endothelial growth factor (VEGF) antibody bevacizumab. Patients were eligible for inclusion in the study if they were 20 years or older, diagnosed with NSCLC, and received docetaxel monotherapy alone or in combination with bevacizumab at the Department of Respiratory Medicine, Kobe City Medical Center General Hospital, between July 1, 2011, and March 31, 2018. Eighty-one patients with recurrent or advanced NSCLC were included. Multivariate stepwise logistic regression analysis with backward selection revealed that lower baseline Eastern Cooperative Oncology Group performance status (ECOG-PS) scores of 1 and 2 (odds ratio (OR), 5.098; 95% confidence interval (CI), 1.045–24.879, p = 0.021) and baseline platelet count below 18.8 × 104/µL (OR, 3.861; 95% CI, 1.211–12.311, p = 0.022) were significant factors influencing the FN occurrence rate. Our results demonstrated that ECOG-PS 1–2 and lower baseline platelet count were significant risk factors of FN in patients with NSCLC receiving docetaxel-based chemotherapy. Moreover, the combination of anti-VEGF antibodies and docetaxel might be associated with increased FN frequency. Despite the limitations of this study including its retrospective design, single-center site, and small sample size, baseline ECOG-PS score and platelet count may be regarded as important indices to identify patients for prophylactic granulocyte-colony stimulating factor (G-CSF) treatment before docetaxel-based chemotherapy.