- 著者
- Mayako Uchida Yasuhiro Mori Kenta Akiba Moena Miyasaka Tatsuya Hirano Hiroaki Ikesue Yuki Yamaguchi Aoi Takano Nami Maegawa Yoshimitsu Shimomura Keiko Hosohata Nobuyuki Muroi Takayuki Ishikawa Tohru Hashida Tsutomu Nakamura
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.43, no.10, pp.1577-1582, 2020-10-01 (Released:2020-10-01)
- 参考文献数
- 26
- 被引用文献数
- 2
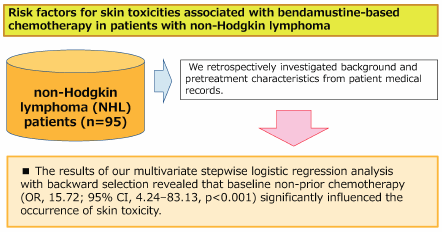
Bendamustine plays an especially important role as a treatment for non-Hodgkin lymphoma (NHL). However, patients administered bendamustine alone or in combination with rituximab (BR) may experience drug-associated skin toxicities that can profoundly impact their health-related QOL through both physical discomfort and psychological distress. Moreover, worsening skin symptoms may lead to dose reduction or termination in the management of cancer chemotherapy. We retrospectively investigated patient backgrounds and pretreatment characteristics from medical records of NHL patients treated with bendamustine alone or BR therapy and identified predictive factors for skin toxicities at the start of chemotherapy. Patients were eligible for the study if they were 20 years older, diagnosed with NHL, and received bendamustine alone or BR therapy at the Department of Hematology, Kobe City Medical Center General Hospital, between April 1, 2011, and March 31, 2018. This study included 95 patients with newly diagnosed or refractory or relapsed NHL. Multivariate stepwise logistic regression analysis with backward selection revealed that baseline non-prior chemotherapy (odds ratio (OR), 15.72; 95% confidence interval (CI), 4.24–83.13, p < 0.001) was a significant factor influencing the occurrence of skin toxicity. Our results demonstrated that non-prior chemotherapy was a significant risk factor for skin toxicities in patients with NHL receiving bendamustine alone or BR therapy. No patient experience serious side effects of grade 3 or higher and that bendamustine is very useful as a first-line treatment.
- 著者
- Tatsuya Hirano Tomohiko Kinoshita Daichi Kazamori Satoshi Inoue Kouji Nishimura Asuka Sakurai Kensuke Ohishi Yasuhiro Kuramoto Hirotaka Amano Akira Yazaki
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.66, no.3, pp.235-238, 2018-03-01 (Released:2018-03-01)
- 参考文献数
- 25
- 被引用文献数
- 5
WFQ-101 with a unique N-1 substituent, 5-amino-4-fluoro-2-(hydroxymethyl)phenyl group, was selected as a lead compound through combination screening based on antimicrobial activity and the efflux index against quinolone-resistant (QR) Pseudomonas aeruginosa (P. aeruginosa). Through structural optimization, we identified WFQ-228 as a novel fluoroquinolone antibiotic candidate. WFQ-228 had potent and superior activity in comparison to levofloxacin (LVX) and ciprofloxacin (CIP) against clinical isolates of P. aeruginosa, Escherichia coli and Acinetobacter baumannii, including QR strains. Furthermore, WFQ-228 demonstrated the potential to overcome major mechanisms of drug resistance; its antimicrobial activity was less affected by both pump-mediated efflux and mutations of the quinolone resistance-determining region in P. aeruginosa compared with LVX and CIP. These results suggest that WFQ-228 is a promising candidate for further evaluation in the treatment of infections caused by QR Gram-negative pathogens.