- 著者
- Hiroaki Takemoto Jun Takahashi Sumiko Hyuga Hiroshi Odaguchi Nahoko Uchiyama Takuro Maruyama Tadatoshi Yamashita Masashi Hyuga Naohiro Oshima Yoshiaki Amakura Takashi Hakamatsuka Yukihiro Goda Toshihiko Hanawa Yoshinori Kobayashi
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.41, no.2, pp.247-253, 2018-02-01 (Released:2018-02-01)
- 参考文献数
- 33
- 被引用文献数
- 18
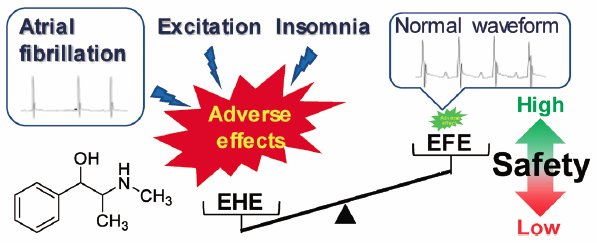
Ephedrine alkaloids-free Ephedra Herb extract (EFE) has been developed to eliminate the adverse effects caused by ephedrine alkaloid-induced sympathetic hyperactivation. Previously, we reported that EFE possesses analgesic, anti-influenza, and cancer metastatic inhibitory effects at comparable levels to that of Ephedra Herb extract (EHE). However, it has not yet been demonstrated that EFE is free from the known side effects of EHE, such as excitation, insomnia, and arrhythmias. In this study, the incidence of these adverse effects was compared between mice administered EHE and those administered EFE. Increased locomotor activity in an open-field test, reduced immobility times in a forced swim test, and reduced sleep times in a pentobarbital-induced sleep test were observed in EHE-treated mice, when compared to the corresponding values in vehicle-treated mice. In contrast, EFE had no obvious effects in these tests. In electrocardiograms, atrial fibrillation (i.e., irregular heart rhythm, absence of P waves, and appearance of f waves) was observed in the EHE-treated mice. It was suggested that this atrial fibrillation was induced by stimulation of adrenaline β1 receptors, but not by hypokalemia. However, EFE did not affect cardiac electrophysiology. These results suggest that the abovementioned side effects are caused by ephedrine alkaloids in EHE, and that EFE is free from these adverse effects, such as excitation, insomnia, and arrhythmias. Thus, EFE is a promising new botanical drug with few adverse effects.
- 著者
- Yoshiko Furukawa Satoshi Okuyama Yoshiaki Amakura Atsushi Sawamoto Mitsunari Nakajima Morio Yoshimura Michiya Igase Naohiro Fukuda Takahisa Tamai Takashi Yoshida
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.69, no.1, pp.2-10, 2021-01-01 (Released:2021-01-01)
- 参考文献数
- 74
- 被引用文献数
- 7
The elderly experience numerous physiological alterations. In the brain, aging causes degeneration or loss of distinct populations of neurons, resulting in declining cognitive function, locomotor capability, etc. The pathogenic factors of such neurodegeneration are oxidative stress, mitochondrial dysfunction, inflammation, reduced energy homeostatis, decreased levels of neurotrophic factor, etc. On the other hand, numerous studies have investigated various biologically active substances in fruit and vegetables. We focused on the peel of citrus fruit to search for neuroprotective components and found that: 1) 3,5,6,7,8,3′,4′-heptamethoxyflavone (HMF) and auraptene (AUR) in the peel of Kawachi Bankan (Citrus kawachiensis) exert neuroprotective effects; 2) both HMF and AUR can pass through the blood–brain barrier, suggesting that they act directly in the brain; 3) the content of AUR in the peel of K. Bankan was exceptionally high, and consequently the oral administration of the dried peel powder of K. Bankan exerts neuroprotective effects; and 4) intake of K. Bankan juice, which was enriched in AUR by adding peel paste to the raw juice, contributed to the prevention of cognitive dysfunction in aged healthy volunteers. This review summarizes our studies in terms of the isolation/characterization of HMF and AUR in K. Bankan peel, analysis of their actions in the brain, mechanisms of their actions, and trials to develop food that retains their functions.
- 著者
- Shunsuke Nakamori Jun Takahashi Sumiko Hyuga Jinwei Yang Hiroaki Takemoto Takuro Maruyama Naohiro Oshima Nahoko Uchiyama Yoshiaki Amakura Masashi Hyuga Takashi Hakamatsuka Yukihiro Goda Hiroshi Odaguchi Toshihiko Hanawa Yoshinori Kobayashi
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.42, no.9, pp.1538-1544, 2019-09-01 (Released:2019-09-01)
- 参考文献数
- 34
- 被引用文献数
- 12
The analgesic effect of Ephedra Herb (EH) is believed to be derived from the anti-inflammatory action of pseudoephedrine (Pse). We recently reported that ephedrine alkaloids–free EH extract (EFE) attenuates formalin-induced pain to the same level as that achieved by EH extract (EHE), which suggests that the analgesic effect of EH may not be due to ephedrine alkaloids (EAs). To examine the contribution of EAs to the analgesic effect of EH, mice were injected with formalin to induce a biphasic pain reaction (first phase, 0–5 min; second phase, 10–45 min) at various time points after oral administration of the following test drugs: ephedrine (Eph), Pse, “authentic” EHE from Tsumura & Co. (EHE-Ts), EFE, and EHE that was used as the source of EFE (EHE-To). Biphasic pain was suppressed at 30 min after administration of Eph, EHE-Ts, and EHE-To. At 6 h after administration of EFE, EHE-To, and Pse—and at 4 to 6 h after administration of EHE-Ts—only second-phase pain was suppressed; however, the effect of Pse at 6 h was not significant. These results suggested that EHE has a biphasic analgesic effect against biphasic formalin-induced pain: in the first phase of analgesia (30 min after administration), biphasic pain is suppressed by Eph; in the second phase of analgesia (4–6 h after administration), second-phase pain is alleviated by constituents other than EAs, although Pse may partially contribute to the relief of second-phase pain.
- 著者
- Hideki Sasaki Hiroshi Akiyama Yoshifumi Yoshida Kazunari Kondo Yoshiaki Amakura Yoshimasa Kasahara Tamio Maitani
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.29, no.12, pp.2514-2518, 2006 (Released:2006-12-01)
- 参考文献数
- 21
- 被引用文献数
- 15 20
In autumn 2004, many Japanese patients with renal failure developed cryptogenic encephalopathy by consuming sugihiratake mushroom, a Japanese delicacy. To elucidate the relationship between the cryptogenic cases and this mushroom, we conducted a multivariate analysis of metabolites in ‘Probably Toxic’ sugihiratake collected from the area of encephalopathy outbreaks, and ‘Probably Safe’ sugihiratake collected from unaffected areas using UPLC/ToF MS. The results indicate that the presence of milligram quantities of vitamin D-like compounds per 10 g of dried sugihiratake from the areas of encephalopathy outbreaks. Two hypotheses to induce the encephalopathy are proposed: the found metabolites are (1) vitamin D agonists, which induce acute and severe hypercalcemia and/or hyperammonemia and/or vitamin D toxicity, or (2) vitamin D antagonists, which induce acute and severe hypocalcemia.