- 著者
- Hidetomo Yokoo Eiichi Yamamoto Sayaka Masada Nahoko Uchiyama Genichiro Tsuji Takashi Hakamatsuka Yosuke Demizu Ken-ichi Izutsu Yukihiro Goda
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.69, no.9, pp.872-876, 2021-09-01 (Released:2021-09-01)
- 参考文献数
- 23
- 被引用文献数
- 13
N-Nitrosodimethylamine (NDMA) is a probable human carcinogen. This study investigated the root cause of the presence of NDMA in ranitidine hydrochloride. Forced thermal degradation studies of ranitidine hydrochloride and its inherent impurities (Imps. A, B, C, D, E, F, G, H, I, J, and K) listed in the European and United States Pharmacopeias revealed that in addition to ranitidine, Imps. A, C, D, E, H, and I produce NDMA at different rates in a solid or an oily liquid state. The rate of NDMA formation from amorphous Imps. A, C, and E was 100 times higher than that from crystalline ranitidine hydrochloride under forced degradation at 110 °C for 1 h. Surprisingly, crystalline Imp. H, bearing neither the N,N-dialkyl-2-nitroethene-1,1-diamine moiety nor a dimethylamino group, also generated NDMA in the solid state, while Imp. I, as an oily liquid, favorably produced NDMA at moderate temperatures (e.g., 50 °C). Therefore, strict control of the aforementioned specific impurities in ranitidine hydrochloride during manufacturing and storage allows appropriate control of NDMA in ranitidine and its pharmaceutical products. Understanding the pathways of the stability related NDMA formation enables improved control of the pharmaceuticals to mitigate this risk.
- 著者
- Hiroaki Takemoto Jun Takahashi Sumiko Hyuga Hiroshi Odaguchi Nahoko Uchiyama Takuro Maruyama Tadatoshi Yamashita Masashi Hyuga Naohiro Oshima Yoshiaki Amakura Takashi Hakamatsuka Yukihiro Goda Toshihiko Hanawa Yoshinori Kobayashi
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.41, no.2, pp.247-253, 2018-02-01 (Released:2018-02-01)
- 参考文献数
- 33
- 被引用文献数
- 18
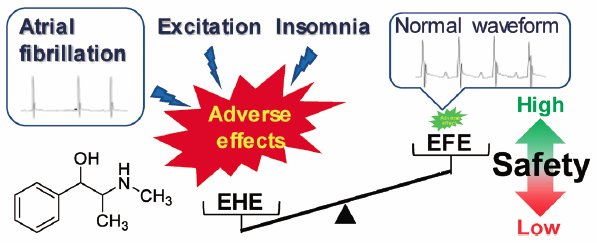
Ephedrine alkaloids-free Ephedra Herb extract (EFE) has been developed to eliminate the adverse effects caused by ephedrine alkaloid-induced sympathetic hyperactivation. Previously, we reported that EFE possesses analgesic, anti-influenza, and cancer metastatic inhibitory effects at comparable levels to that of Ephedra Herb extract (EHE). However, it has not yet been demonstrated that EFE is free from the known side effects of EHE, such as excitation, insomnia, and arrhythmias. In this study, the incidence of these adverse effects was compared between mice administered EHE and those administered EFE. Increased locomotor activity in an open-field test, reduced immobility times in a forced swim test, and reduced sleep times in a pentobarbital-induced sleep test were observed in EHE-treated mice, when compared to the corresponding values in vehicle-treated mice. In contrast, EFE had no obvious effects in these tests. In electrocardiograms, atrial fibrillation (i.e., irregular heart rhythm, absence of P waves, and appearance of f waves) was observed in the EHE-treated mice. It was suggested that this atrial fibrillation was induced by stimulation of adrenaline β1 receptors, but not by hypokalemia. However, EFE did not affect cardiac electrophysiology. These results suggest that the abovementioned side effects are caused by ephedrine alkaloids in EHE, and that EFE is free from these adverse effects, such as excitation, insomnia, and arrhythmias. Thus, EFE is a promising new botanical drug with few adverse effects.
1 0 0 0 OA UHPLC/MS and NMR-Based Metabolomic Analysis of Dried Water Extract of Citrus-Type Crude Drugs
- 著者
- Takashi Tsujimoto Ryoko Arai Taichi Yoshitomi Yutaka Yamamoto Yoshihiro Ozeki Takashi Hakamatsuka Nahoko Uchiyama
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- pp.c21-00180, (Released:2021-05-21)
- 参考文献数
- 19
- 被引用文献数
- 9
Citrus-type crude drugs (CCDs) are commonly used to formulate decoctions in Kampo formula (traditional Japanese medicine). Our previous study reported metabolomic analyses for differentiation of the methanol extracts of Citrus-type crude drugs (CCDs) using UHPLC/MS, and 13C- and 1H-NMR. The present study expanded the scope of its application by analyzing four CCD water extracts (Kijitsu, Tohi, Chimpi, and Kippi); these CCDs are usually used as decoction ingredients in the Kampo formula. A principal component analysis score plot of processed UPLC/MS and NMR analysis data indicated that the CCD water extracts could be classified into three groups. The loading plots showed that naringin and neohesperidin were the distinguishing components. Three primary metabolites, α-glucose, β-glucose, and sucrose were identified as distinguishing compounds by NMR spectroscopy. During the preparation of CCD dry extracts, some compounds volatilized or decomposed. Consequently, fewer compounds were detected than in our previous studies using methanol extract. However, these results suggested that the combined NMR- and LC/MS-based metabolomics can discriminate crude drugs in dried water extracts of CCDs.
- 著者
- Yasuhiro Abe Eiichi Yamamoto Hiroyuki Yoshida Akiko Usui Naomi Tomita Hitomi Kanno Sayaka Masada Hidetomo Yokoo Genichiro Tsuji Nahoko Uchiyama Takashi Hakamatsuka Yosuke Demizu Ken-ichi Izutsu Yukihiro Goda Haruhiro Okuda
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- pp.c20-00431, (Released:2020-08-08)
- 参考文献数
- 28
- 被引用文献数
- 20
The purpose of this study was to elucidate the effect of high-temperature storage on the stability of ranitidine, specifically with respect to the potential formation of N-nitrosodimethylamine (NDMA), which is classified as a probable human carcinogen. Commercially available ranitidine reagent powders and formulations were stored under various conditions, and subjected to LC-MS/MS analysis. When ranitidine tablets from two different brands (designated as tablet A and tablet B) were stored under accelerated condition (40°C with 75% relative humidity), following the drug stability guidelines issued by the International Conference on Harmonisation (ICH-Q1A), for up to 8 weeks, the amount of NDMA in them substantially increased from 0.19 to 116 ppm and from 2.89 to 18 ppm, respectively. The formation of NDMA that exceeded the acceptable daily intake limit (0.32 ppm) at the temperature used under accelerated storage conditions clearly highlights the risk of NDMA formation in ranitidine formulations when extrapolated to storage under ambient conditions. A forced-degradation study under the stress condition (60°C for 1 week) strongly suggested that environmental factors such as moisture and oxygen are involved in the formation of NDMA in ranitidine formulations. Storage of ranitidine tablets and reagent powders at the high temperatures also increased the amount of nitrite, which is considered one of the factors influencing NDMA formation. These data indicate the necessity of controlling/monitoring stability-related factors, in addition to controlling impurities during the manufacturing process, in order to mitigate nitrosamine-related health risks of certain pharmaceuticals.
- 著者
- Shunsuke Nakamori Jun Takahashi Sumiko Hyuga Jinwei Yang Hiroaki Takemoto Takuro Maruyama Naohiro Oshima Nahoko Uchiyama Yoshiaki Amakura Masashi Hyuga Takashi Hakamatsuka Yukihiro Goda Hiroshi Odaguchi Toshihiko Hanawa Yoshinori Kobayashi
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.42, no.9, pp.1538-1544, 2019-09-01 (Released:2019-09-01)
- 参考文献数
- 34
- 被引用文献数
- 12
The analgesic effect of Ephedra Herb (EH) is believed to be derived from the anti-inflammatory action of pseudoephedrine (Pse). We recently reported that ephedrine alkaloids–free EH extract (EFE) attenuates formalin-induced pain to the same level as that achieved by EH extract (EHE), which suggests that the analgesic effect of EH may not be due to ephedrine alkaloids (EAs). To examine the contribution of EAs to the analgesic effect of EH, mice were injected with formalin to induce a biphasic pain reaction (first phase, 0–5 min; second phase, 10–45 min) at various time points after oral administration of the following test drugs: ephedrine (Eph), Pse, “authentic” EHE from Tsumura & Co. (EHE-Ts), EFE, and EHE that was used as the source of EFE (EHE-To). Biphasic pain was suppressed at 30 min after administration of Eph, EHE-Ts, and EHE-To. At 6 h after administration of EFE, EHE-To, and Pse—and at 4 to 6 h after administration of EHE-Ts—only second-phase pain was suppressed; however, the effect of Pse at 6 h was not significant. These results suggested that EHE has a biphasic analgesic effect against biphasic formalin-induced pain: in the first phase of analgesia (30 min after administration), biphasic pain is suppressed by Eph; in the second phase of analgesia (4–6 h after administration), second-phase pain is alleviated by constituents other than EAs, although Pse may partially contribute to the relief of second-phase pain.
1 0 0 0 OA Evaluation of the Botanical Origin of Black Cohosh Products by Genetic and Chemical Analyses
- 著者
- Sayaka Masada-Atsumi Yukie Kumeta Yutaka Takahashi Takashi Hakamatsuka Yukihiro Goda
- 出版者
- 公益社団法人日本薬学会
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.37, no.3, pp.454-460, 2014-03-01 (Released:2014-03-01)
- 参考文献数
- 23
- 被引用文献数
- 5 18 3
Despite the increasing sales of black cohosh (the dried rhizome and root of Cimicifuga racemosa L.) in the world herbal market, these products have continuous adulteration issues. The botanical authenticity of the black cohosh products is the first important step for ensuring their quality, safety and efficacy. In this study, we genetically identified the botanical sources of 10 black cohosh products and 5 Cimicifuga Rhizome crude drugs of Japanese Pharmacopoeia grade, and analyzed the metabolic profiling of 25 black cohosh products using liquid chromatography-tandem mass spectrometry (LC-MS/MS). Consequently, we found that C. dahurica and possibly C. foetida are misused as sources of the black cohosh products and in some cases, the extracts of black cohosh were adulterated with the plant materials of C. dahurica. We demonstrated that these three species can be distinguished by three marker compounds in a specific mass range. These results must be helpful in establishing regulations for the safe use of the black cohosh products.