- 著者
- Akihiko Nogami Takashi Kurita Haruhiko Abe Kenji Ando Toshiyuki Ishikawa Katsuhiko Imai Akihiko Usui Kaoru Okishige Kengo Kusano Koichiro Kumagai Masahiko Goya Yoshinori Kobayashi Akihiko Shimizu Wataru Shimizu Morio Shoda Naokata Sumitomo Yoshihiro Seo Atsushi Takahashi Hiroshi Tada Shigeto Naito Yuji Nakazato Takashi Nishimura Takashi Nitta Shinichi Niwano Nobuhisa Hagiwara Yuji Murakawa Teiichi Yamane Takeshi Aiba Koichi Inoue Yuki Iwasaki Yasuya Inden Kikuya Uno Michio Ogano Masaomi Kimura Shun-ichiro Sakamoto Shingo Sasaki Kazuhiro Satomi Tsuyoshi Shiga Tsugutoshi Suzuki Yukio Sekiguchi Kyoko Soejima Masahiko Takagi Masaomi Chinushi Nobuhiro Nishi Takashi Noda Hitoshi Hachiya Masataka Mitsuno Takeshi Mitsuhashi Yasushi Miyauchi Aya Miyazaki Tomoshige Morimoto Hiro Yamasaki Yoshifusa Aizawa Tohru Ohe Takeshi Kimura Kazuo Tanemoto Hiroyuki Tsutsui Hideo Mitamura on behalf of the JCS/JHRS Joint Working Group
- 出版者
- The Japanese Circulation Society
- 雑誌
- Circulation Journal (ISSN:13469843)
- 巻号頁・発行日
- pp.CJ-20-0637, (Released:2021-06-01)
- 参考文献数
- 1350
- 被引用文献数
- 75
- 著者
- Hiroaki Takemoto Jun Takahashi Sumiko Hyuga Hiroshi Odaguchi Nahoko Uchiyama Takuro Maruyama Tadatoshi Yamashita Masashi Hyuga Naohiro Oshima Yoshiaki Amakura Takashi Hakamatsuka Yukihiro Goda Toshihiko Hanawa Yoshinori Kobayashi
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.41, no.2, pp.247-253, 2018-02-01 (Released:2018-02-01)
- 参考文献数
- 33
- 被引用文献数
- 18
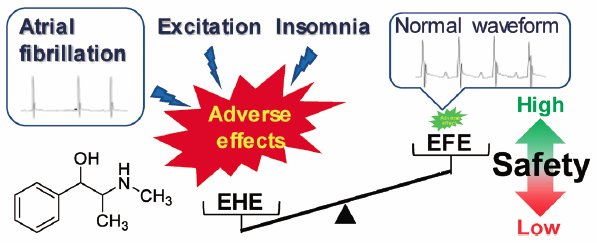
Ephedrine alkaloids-free Ephedra Herb extract (EFE) has been developed to eliminate the adverse effects caused by ephedrine alkaloid-induced sympathetic hyperactivation. Previously, we reported that EFE possesses analgesic, anti-influenza, and cancer metastatic inhibitory effects at comparable levels to that of Ephedra Herb extract (EHE). However, it has not yet been demonstrated that EFE is free from the known side effects of EHE, such as excitation, insomnia, and arrhythmias. In this study, the incidence of these adverse effects was compared between mice administered EHE and those administered EFE. Increased locomotor activity in an open-field test, reduced immobility times in a forced swim test, and reduced sleep times in a pentobarbital-induced sleep test were observed in EHE-treated mice, when compared to the corresponding values in vehicle-treated mice. In contrast, EFE had no obvious effects in these tests. In electrocardiograms, atrial fibrillation (i.e., irregular heart rhythm, absence of P waves, and appearance of f waves) was observed in the EHE-treated mice. It was suggested that this atrial fibrillation was induced by stimulation of adrenaline β1 receptors, but not by hypokalemia. However, EFE did not affect cardiac electrophysiology. These results suggest that the abovementioned side effects are caused by ephedrine alkaloids in EHE, and that EFE is free from these adverse effects, such as excitation, insomnia, and arrhythmias. Thus, EFE is a promising new botanical drug with few adverse effects.
- 著者
- Katsushige Ono Yu-ki Iwasaki Masaharu Akao Takanori Ikeda Kuniaki Ishii Yasuya Inden Kengo Kusano Yoshinori Kobayashi Yukihiro Koretsune Tetsuo Sasano Naokata Sumitomo Naohiko Takahashi Shinichi Niwano Nobuhisa Hagiwara Ichiro Hisatome Tetsushi Furukawa Haruo Honjo Toru Maruyama Yuji Murakawa Masahiro Yasaka Eiichi Watanabe Takeshi Aiba Mari Amino Hideki Itoh Hisashi Ogawa Yasuo Okumura Chizuko Aoki-Kamiya Jun Kishihara Eitaro Kodani Takashi Komatsu Yusuke Sakamoto Kazuhiro Satomi Tsuyoshi Shiga Tetsuji Shinohara Atsushi Suzuki Shinya Suzuki Yukio Sekiguchi Satoshi Nagase Noriyuki Hayami Masahide Harada Tadashi Fujino Takeru Makiyama Mitsunori Maruyama Junichiro Miake Shota Muraji Hiroshige Murata Norishige Morita Hisashi Yokoshiki Koichiro Yoshioka Kenji Yodogawa Hiroshi Inoue Ken Okumura Takeshi Kimura Hiroyuki Tsutsui Wataru Shimizu on behalf of the Japanese Circulation Society and Japanese Heart Rhythm Society Joint Working Group
- 出版者
- The Japanese Circulation Society
- 雑誌
- Circulation Journal (ISSN:13469843)
- 巻号頁・発行日
- pp.CJ-20-1212, (Released:2022-03-11)
- 参考文献数
- 998
- 被引用文献数
- 44
- 著者
- Ken Okumura Kazuo Matsumoto Yoshinori Kobayashi Akihiko Nogami Robert B Hokanson Fred Kueffer for the CRYO-Japan PMS Study Investigators
- 出版者
- 日本循環器学会
- 雑誌
- Circulation Journal (ISSN:13469843)
- 巻号頁・発行日
- pp.CJ-16-0285, (Released:2016-06-30)
- 参考文献数
- 17
- 被引用文献数
- 2 31
Background:Outcomes of cryoballoon ablation for paroxysmal atrial fibrillation (PAF) have been reported in the Western countries but not in Japan. The CRYO-Japan PMS study was a single-arm, observational, multicenter, prospective study of the 2nd-generation cryoballoon Arctic Front AdvanceTM. We evaluated device- and procedure-related complications and clinical outcomes at 6 months.Methods and Results:The 616 patients (male, 72%; mean age, 63±11 years) were enrolled from 33 Japanese hospitals. Of all patients, 610 had PAF, and procedural data were analyzed in 607. A subset of 328 patients was followed for 6 months for the primary efficacy analysis. AF recurrence outside the 3-month blanking period or repeat ablation was considered treatment failure. Pulmonary vein isolation was achieved in 606/607 patients (99.8%); 1 patient (0.3%) had a repeat ablation during the blanking period. Freedom from AF at 6 months was 88.4% (95% CI: 84.1–91.6%). Device- and/or procedure-related adverse events included phrenic nerve injury unresolved at hospital discharge in 9/616 patients (1.5%), which resolved within 6 months in 7, pericardial effusion in 5/616 (0.8%), and tamponade in 4/616 (0.6%). One non-device-related death from pneumonia was reported 6 days post-procedure.Conclusions:Cryoballoon ablation is safe and effective for Japanese PAF patients, with 88.4% AF freedom at 6 months post-ablation.
- 著者
- Shunsuke Nakamori Jun Takahashi Sumiko Hyuga Jinwei Yang Hiroaki Takemoto Takuro Maruyama Naohiro Oshima Nahoko Uchiyama Yoshiaki Amakura Masashi Hyuga Takashi Hakamatsuka Yukihiro Goda Hiroshi Odaguchi Toshihiko Hanawa Yoshinori Kobayashi
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.42, no.9, pp.1538-1544, 2019-09-01 (Released:2019-09-01)
- 参考文献数
- 34
- 被引用文献数
- 12
The analgesic effect of Ephedra Herb (EH) is believed to be derived from the anti-inflammatory action of pseudoephedrine (Pse). We recently reported that ephedrine alkaloids–free EH extract (EFE) attenuates formalin-induced pain to the same level as that achieved by EH extract (EHE), which suggests that the analgesic effect of EH may not be due to ephedrine alkaloids (EAs). To examine the contribution of EAs to the analgesic effect of EH, mice were injected with formalin to induce a biphasic pain reaction (first phase, 0–5 min; second phase, 10–45 min) at various time points after oral administration of the following test drugs: ephedrine (Eph), Pse, “authentic” EHE from Tsumura & Co. (EHE-Ts), EFE, and EHE that was used as the source of EFE (EHE-To). Biphasic pain was suppressed at 30 min after administration of Eph, EHE-Ts, and EHE-To. At 6 h after administration of EFE, EHE-To, and Pse—and at 4 to 6 h after administration of EHE-Ts—only second-phase pain was suppressed; however, the effect of Pse at 6 h was not significant. These results suggested that EHE has a biphasic analgesic effect against biphasic formalin-induced pain: in the first phase of analgesia (30 min after administration), biphasic pain is suppressed by Eph; in the second phase of analgesia (4–6 h after administration), second-phase pain is alleviated by constituents other than EAs, although Pse may partially contribute to the relief of second-phase pain.